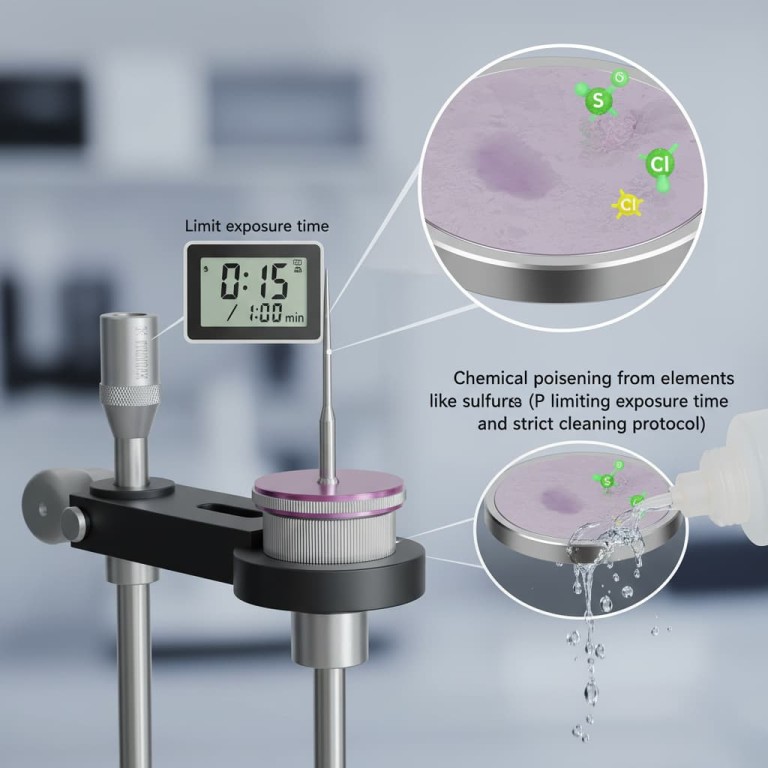
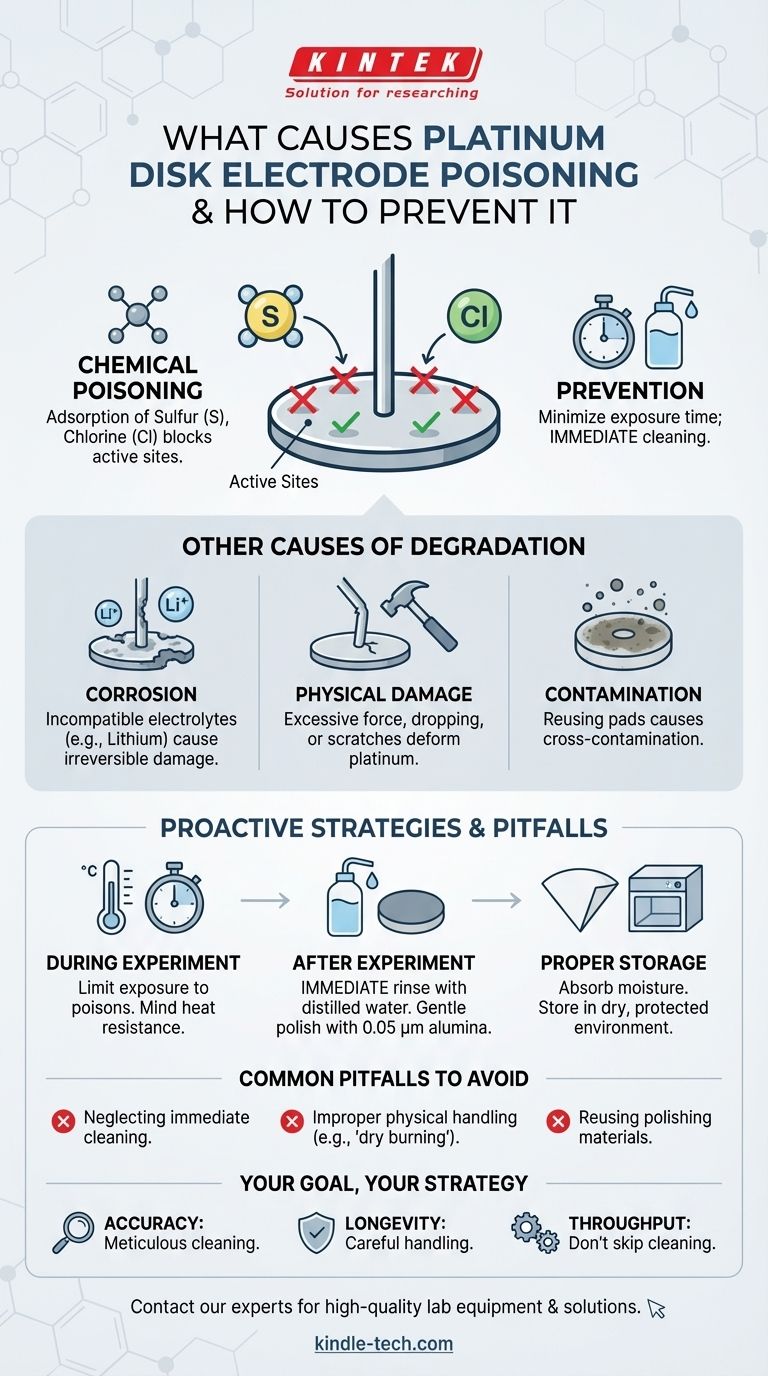
Poisoning of a platinum disk electrode is primarily caused by chemical adsorption from elements like sulfur and chlorine. When present in an electrolyte, these substances bind to the platinum surface, blocking active sites and causing a significant decline in electrochemical performance. Prevention hinges on minimizing the electrode's exposure time to these electrolytes and executing a strict cleaning protocol immediately after use.
The core issue extends beyond simple chemical "poisoning." Maintaining a high-performance electrode requires a holistic approach that combines careful chemical selection with rigorous protocols for physical handling, cleaning, and storage to ensure the integrity of your results.
Unpacking the Causes of Electrode Degradation
An electrode's failure is rarely due to a single event. It is typically the result of chemical exposure, physical mishandling, or improper maintenance, all of which compromise the sensitive platinum surface.
Chemical Poisoning: The Primary Culprit
The term poisoning specifically refers to the deactivation of the electrode's catalytic surface.
Elements like sulfur and chlorine have a strong affinity for platinum. They adsorb onto the disk, effectively preventing the desired electrochemical reactions from occurring and leading to inaccurate measurements.
Corrosion from Incompatible Electrolytes
Beyond poisoning, outright corrosion can occur if the electrolyte is incompatible with platinum.
For example, certain experiments involving lithium can be particularly aggressive towards platinum, causing irreversible damage. Always verify that your chosen electrolyte and experimental conditions are suitable for the electrode material.
Physical Damage and Deformation
Platinum is a relatively soft metal, making the electrode highly susceptible to physical damage.
Applying excessive force, dropping the electrode, or allowing it to collide with hard objects can deform the platinum wire or scratch the disk surface, altering its electrochemical properties.
Contamination During Maintenance
Improper cleaning can introduce more problems than it solves.
Using the same polishing pad for different polishing powders is a common source of cross-contamination. This embeds foreign particles into the platinum surface, creating unpredictable variables in future experiments.
Proactive Strategies for Prevention and Maintenance
A disciplined maintenance routine is the most effective way to protect your investment and ensure the reliability of your data.
During the Experiment: Mind Your Conditions
The best defense is avoiding damaging conditions in the first place.
Limit the electrode's exposure time in electrolytes known to contain poisons. Furthermore, ensure that high-temperature experiments do not exceed the electrode's specified heat resistance to prevent permanent damage.
After the Experiment: The Cleaning Protocol
Cleaning should be performed immediately after the experiment concludes.
First, remove the electrode from the electrolyte and rinse it thoroughly with distilled water. This removes any residual solution and impurities before they can cause further harm.
For stubborn residues, you can gently polish the surface using a dedicated pad with 0.05 µm alumina powder.
Proper Storage for Longevity
How you store the electrode is just as important as how you use it.
After cleaning, carefully absorb any surface moisture with filter paper. Store the electrode in a dry environment, protecting it from humidity, high temperatures, and strong light.
Common Pitfalls and How to Avoid Them
Even experienced researchers can fall into habits that degrade electrode performance over time. Understanding these common mistakes is critical for prevention.
Neglecting Immediate Cleaning
Leaving an electrode to sit in solution or even air-dry with residual electrolyte is a critical error. This allows poisoning and corrosion processes to continue long after the experiment has ended, causing slow but steady degradation.
Improper Physical Handling
Treating the electrode like a robust piece of hardware is a recipe for failure. Never apply current without an electrolyte (a practice known as dry burning) and always handle it with care to avoid mechanical shock or impact.
Reusing Polishing Materials
Attempting to save time or materials by reusing polishing pads is a false economy. The risk of cross-contamination is extremely high and can invalidate entire sets of future experimental data by introducing unknown catalytic or interfering agents.
Making the Right Choice for Your Goal
Your maintenance strategy should align directly with your experimental priorities.
- If your primary focus is analytical accuracy: Meticulous cleaning, avoiding cross-contamination by using dedicated polishing pads, and immediate rinsing are non-negotiable.
- If your primary focus is electrode longevity: Careful physical handling to prevent scratches or deformation and proper dry storage are the most critical factors.
- If your primary focus is experimental throughput: Skipping the immediate post-experiment cleaning protocol is a costly mistake that will lead to performance decline, data unreliability, and eventual electrode failure.
A disciplined approach to electrode care is the foundation of trustworthy electrochemical research.
Summary Table:
| Cause of Degradation | Primary Prevention Method |
|---|---|
| Chemical Poisoning (S, Cl) | Limit exposure time; immediate cleaning |
| Corrosion (e.g., Li electrolytes) | Verify electrolyte compatibility |
| Physical Damage (Scratches) | Handle with care; avoid impact |
| Contamination (Polishing) | Use dedicated pads for each powder |
Ensure your electrochemical experiments are built on a foundation of reliable data. Proper electrode maintenance is critical, but having the right equipment is just as important. KINTEK specializes in high-quality lab equipment and consumables, including electrodes and cleaning supplies, to serve all your laboratory needs.
Let us help you achieve consistent, accurate results. Contact our experts today to find the perfect solutions for your research!
Visual Guide
Related Products
- Platinum Sheet Electrode for Battery Lab Applications
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Electrolytic Electrochemical Cell for Coating Evaluation
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What are the performance characteristics of platinum wire/rod electrodes? Unmatched Stability for Your Lab
- How should a platinum wire/rod electrode be installed? Ensure Accurate Electrochemical Measurements
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- How can a worn or scratched platinum disk electrode surface be restored? Achieve a Mirror Finish for Reliable Data
- What are platinum electrodes used for? Essential Uses in Science, Medicine, and Industry



















