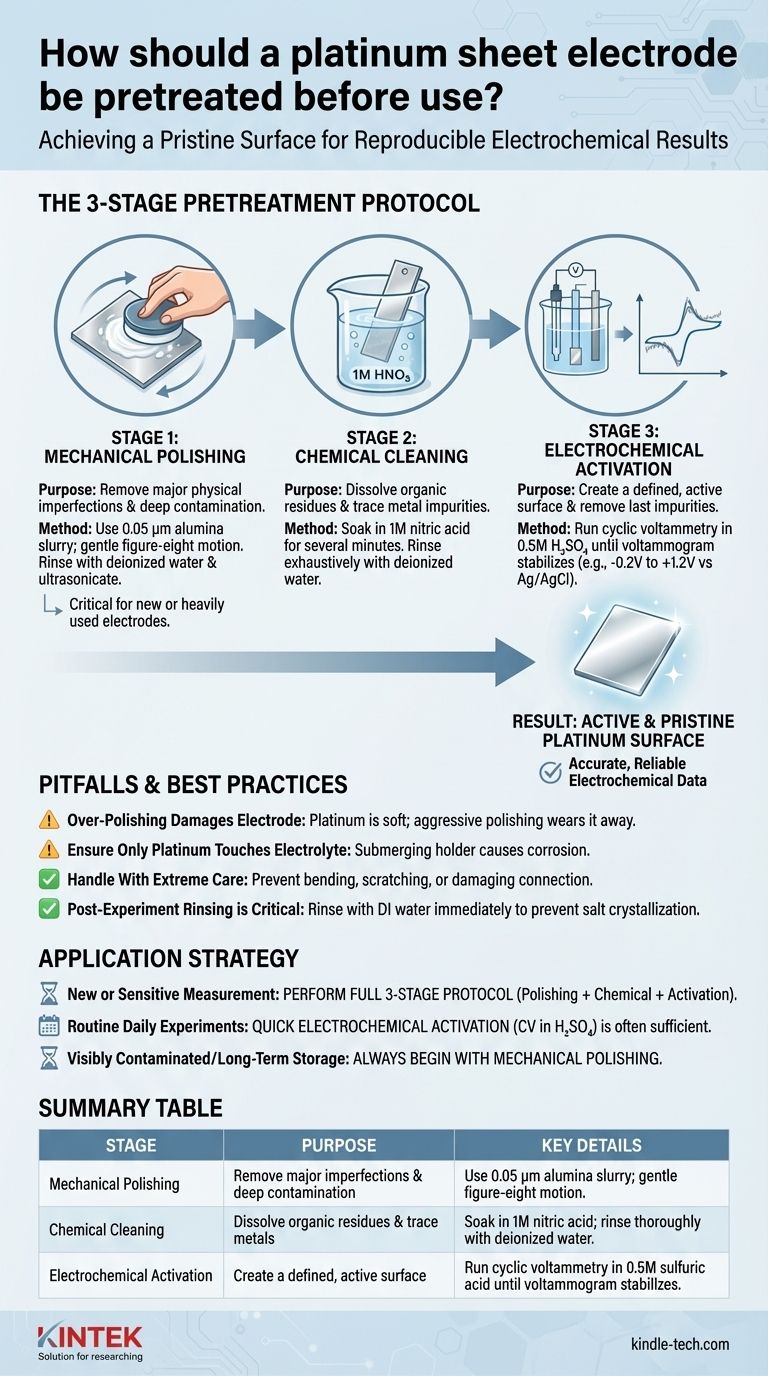
To properly pretreat a platinum sheet electrode, you must perform a sequence of cleaning and activation steps to remove physical and chemical contaminants. The most comprehensive method involves mechanical polishing with alumina powder, followed by chemical washing in nitric acid, and finally, electrochemical cleaning using cyclic voltammetry in a sulfuric acid solution until a stable voltammogram is achieved.
The goal of pretreatment is not simply to clean the electrode, but to create a reproducibly active and pristine platinum surface. This ensures the accuracy and reliability of your electrochemical measurements, as even trace contaminants can dramatically alter experimental results.

The Purpose of Pretreatment: Achieving a Pristine Surface
Why a Clean Surface is Non-Negotiable
A platinum electrode's effectiveness hinges on the purity of its surface. Any organic residue, adsorbed ions, or surface oxides from previous experiments or storage can block active sites.
This contamination interferes with electron transfer kinetics and can introduce unwanted side reactions, leading to inaccurate, noisy, or entirely incorrect data.
The Role of Platinum Purity
High-quality platinum sheet electrodes are typically made of 99.99% pure platinum. This inherent purity is the foundation for accurate measurements.
Pretreatment is the process of restoring the working surface to this pristine state, ensuring that your experiment interacts with pure platinum, not a layer of unknown contaminants.
The Standard Pretreatment Protocol
For the most reliable results, especially in sensitive experiments, a three-stage protocol is recommended. These steps should be performed in sequence.
Stage 1: Mechanical Polishing
This step is designed to remove major physical imperfections, deep-seated contamination, and thick oxide layers. It is not always necessary for routine use but is critical for restoring a heavily used or new electrode.
To perform mechanical polishing, create a slurry of fine alumina powder (e.g., 0.05 µm) and deionized water on a polishing pad. Gently polish the electrode in a figure-eight motion, then rinse it thoroughly with deionized water. Ultrasonication in deionized water is often used to remove any residual polishing particles.
Stage 2: Chemical Cleaning
Chemical cleaning dissolves organic residues and trace metal impurities that mechanical polishing may miss.
Soak the electrode in a dilute acid, such as 1M nitric acid (HNO₃), for several minutes. Nitric acid is an excellent choice as it is a strong oxidizing agent that effectively removes organic contaminants without leaving behind interfering ions (like chloride from HCl). After soaking, rinse the electrode exhaustively with deionized water.
Stage 3: Electrochemical Activation
This is the final and most important step. It removes the last traces of impurities and creates a well-defined, active platinum surface. This is typically done in the actual electrochemical cell or a dedicated cleaning cell.
The standard method is to run cyclic voltammetry (CV) on the electrode in a deaerated 0.5M sulfuric acid (H₂SO₄) solution. Scan the potential between the onset of hydrogen and oxygen evolution (e.g., -0.2 V to +1.2 V vs. Ag/AgCl) until the voltammogram becomes stable and exhibits the characteristic peaks for hydrogen adsorption/desorption and platinum oxide formation/reduction. A stable, textbook-perfect voltammogram is the definitive sign of a clean platinum surface.
Understanding the Pitfalls and Best Practices
Achieving a clean surface requires careful technique and an awareness of potential problems.
Over-Polishing Can Damage the Electrode
Platinum is a soft metal. Aggressive or frequent mechanical polishing will slowly wear away the electrode material, reducing its lifespan. Use polishing only when necessary to remove visible scratches or stubborn contamination.
Ensure Only Platinum Touches the Electrolyte
The electrode is designed so that only the platinum sheet itself should be immersed in the electrolyte solution. Submerging the connection point or other parts of the holder will lead to corrosion and contamination of your experiment.
Handle With Extreme Care
Beyond being soft, platinum electrodes are expensive and can be fragile. Always handle them gently to prevent bending, scratching, or damaging the connection between the sheet and the holder.
Post-Experiment Rinsing is Critical
Immediately after an experiment, rinse the electrode thoroughly with deionized water. This prevents the electrolyte from drying on the surface, which can cause salt crystallization and make future cleaning more difficult. For long-term storage, ensure it is completely dry and kept in a clean, dedicated container.
How to Apply This to Your Experiment
Your pretreatment strategy depends on the state of your electrode and the demands of your experiment.
- If you are preparing for a new or highly sensitive measurement: Perform the full three-stage protocol—mechanical polishing, chemical cleaning, and electrochemical activation—to ensure the most pristine surface possible.
- If you are running routine daily experiments: A quick electrochemical activation cycle in sulfuric acid before your first measurement is often sufficient to refresh a well-maintained electrode.
- If the electrode is visibly contaminated, scratched, or has been in long-term storage: Always begin with mechanical polishing to reset the surface before proceeding with chemical and electrochemical cleaning.
Ultimately, consistent and meticulous pretreatment is the foundation for producing high-quality, reproducible electrochemical data.
Summary Table:
| Pretreatment Stage | Purpose | Key Details |
|---|---|---|
| Mechanical Polishing | Remove major imperfections & deep contamination | Use 0.05 µm alumina slurry; gentle figure-eight motion. |
| Chemical Cleaning | Dissolve organic residues & trace metals | Soak in 1M nitric acid; rinse thoroughly with deionized water. |
| Electrochemical Activation | Create a defined, active surface | Run cyclic voltammetry in 0.5M sulfuric acid until voltammogram stabilizes. |
Achieve Reproducible Electrochemical Results with KINTEK
Proper electrode pretreatment is fundamental to the success of your experiments. KINTEK specializes in providing high-purity lab equipment and consumables, including the platinum electrodes and essential cleaning supplies mentioned in this guide, to support your laboratory's precision needs.
Let our experts help you select the right materials and optimize your protocols for consistent, high-quality data. Contact our team today to discuss your specific electrode requirements and ensure your lab is equipped for success.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
People Also Ask
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- How should a platinum sheet electrode be operated during an experiment? Ensure Accurate and Reproducible Results
- What is the proper post-treatment procedure for a platinum sheet electrode? Ensure Long-Term Accuracy & Protect Your Investment



















