At their core, platinum sheet electrodes are defined by three primary performance characteristics: exceptional chemical stability, high catalytic activity for key electrochemical reactions, and a wide, stable operating potential window. This combination makes them a benchmark standard for a vast range of electrochemical experiments, particularly where reliability and non-interference are paramount.
The true value of a platinum sheet electrode lies not just in its individual properties, but in its ability to provide a stable, reproducible, and well-defined surface. This allows researchers to isolate and study their reaction of interest with confidence, minimizing the electrode itself as a variable.

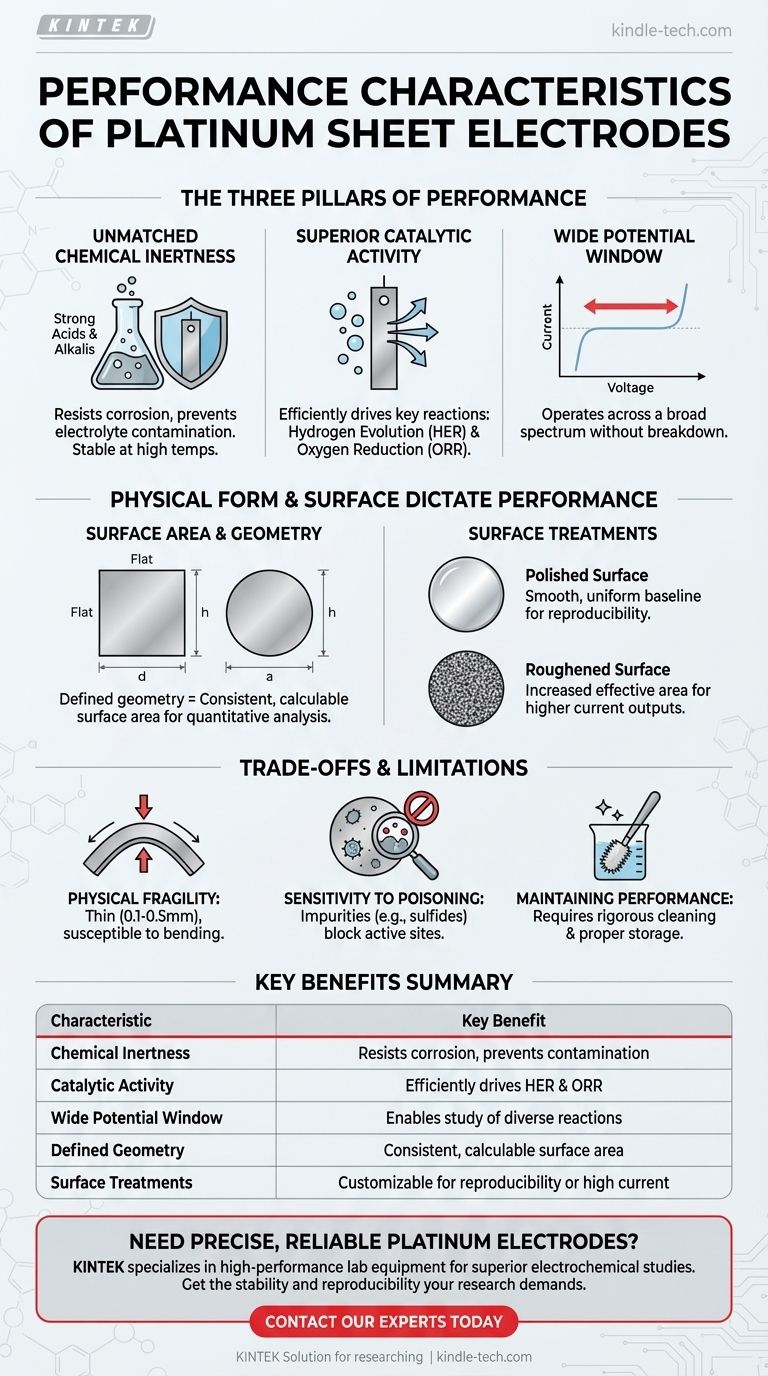
The Pillars of Platinum Electrode Performance
The utility of a platinum sheet electrode is built upon a foundation of distinct chemical and electrical properties. Understanding these pillars is key to leveraging the electrode effectively.
Unmatched Chemical Inertness
Platinum exhibits remarkable chemical inertness. It is highly resistant to corrosion from strong acids (like hydrochloric, sulfuric, and nitric acid), strong alkalis, and degradation at high temperatures.
This stability is critical because it ensures the electrode does not dissolve or react with the electrolyte, which would introduce contaminants and interfere with the accuracy of your experiment.
Superior Catalytic Activity
Beyond being inert, platinum is an excellent catalyst. It is particularly effective at facilitating important electrochemical reactions like the hydrogen evolution reaction (HER) and the oxygen reduction reaction (ORR).
This catalytic performance means that these reactions can occur more efficiently and at lower energy costs on the platinum surface, making it an ideal material for studying or driving these specific processes.
Wide Electrochemical Potential Window
Platinum can operate across a very wide potential range without undergoing oxidation or reduction itself.
This characteristic is invaluable in electrochemistry, as it allows researchers to study a broad spectrum of reactions without the risk of the electrode material breaking down and compromising the results.
How Physical Form Dictates Performance
The performance of a platinum electrode is not just a function of its material properties but is also directly influenced by its physical shape and surface condition.
The Role of Surface Area and Geometry
The defined, flat geometry of a platinum sheet (available in square, circular, or rectangular forms) provides a consistent and easily calculable surface area.
This is crucial for quantitative analysis, where measurements like current density (current per unit area) are required for accurate comparison and modeling.
Impact of Surface Treatments
The performance of a platinum sheet can be intentionally modified through surface treatments. A polished surface provides a smooth, uniform baseline, while a roughened surface increases the effective surface area for higher current outputs.
Specialized coatings can also be applied to further enhance catalytic activity or selectivity for specific applications, expanding the electrode's versatility.
Understanding the Trade-offs and Limitations
While platinum is a benchmark material, no tool is without its trade-offs. Acknowledging these limitations is essential for proper application and data interpretation.
Physical Fragility
Platinum sheet electrodes are typically very thin, with thicknesses often ranging from 0.1mm to 0.5mm. This makes them physically fragile and susceptible to bending or damage if not handled with care.
Sensitivity to Surface Contamination
Although chemically robust, the catalytic performance of a platinum surface is highly sensitive to poisoning by impurities in the electrolyte (e.g., sulfides, organic molecules).
This sensitivity necessitates rigorous cleaning protocols to maintain experimental reproducibility, as even trace contaminants can block active sites and alter results.
Maintaining Performance Over Time
Consistent performance is contingent on proper maintenance. The references are clear that without regular care, the electrode's reliability will degrade.
Before each use, the surface must be inspected for contamination and smoothness. After use, it must be rinsed immediately with deionized water, dried, and stored properly to prevent atmospheric contamination and physical damage.
Making the Right Choice for Your Goal
To ensure reliable outcomes, select and prepare your platinum sheet electrode based on your primary experimental objective.
- If your primary focus is fundamental electrochemistry: Use a polished sheet for its well-defined, calculable surface area, ensuring reproducible baseline measurements.
- If your primary focus is high-efficiency catalysis: A roughened or specially coated platinum sheet will provide a higher active surface area for maximizing reaction rates.
- If your primary focus is work in harsh environments: The inherent chemical inertness of platinum makes it the default choice for experiments in aggressive acidic or alkaline media.
Ultimately, understanding these performance characteristics empowers you to use the platinum sheet electrode not just as a component, but as a precise instrument for achieving reliable scientific insight.
Summary Table:
| Characteristic | Key Benefit |
|---|---|
| Chemical Inertness | Resists corrosion in acids/alkalis, prevents contamination |
| Catalytic Activity | Efficiently drives key reactions like HER and ORR |
| Wide Potential Window | Enables study of diverse reactions without electrode breakdown |
| Defined Geometry | Provides consistent, calculable surface area for analysis |
| Surface Treatments | Can be polished for reproducibility or roughened for higher current |
Need precise, reliable platinum electrodes for your lab? KINTEK specializes in high-performance lab equipment and consumables, including platinum sheet electrodes designed for superior electrochemical studies. Our electrodes deliver the chemical stability, catalytic activity, and reproducible results your research demands. Contact our experts today to discuss your specific application and ensure you get the right electrode for your needs!
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Gold Electrochemical Sheet Electrode Gold Electrode
- Gold Disc Electrode
People Also Ask
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- What are the available specifications for platinum sheet electrodes? Find the Perfect Fit for Your Electrochemical Needs
- What precautions should be taken when using a platinum sheet electrode? Ensure Accurate & Reproducible Electrochemical Data
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications
- What is the expected lifespan of a platinum sheet electrode? Maximize Your Electrode's Service Life



















