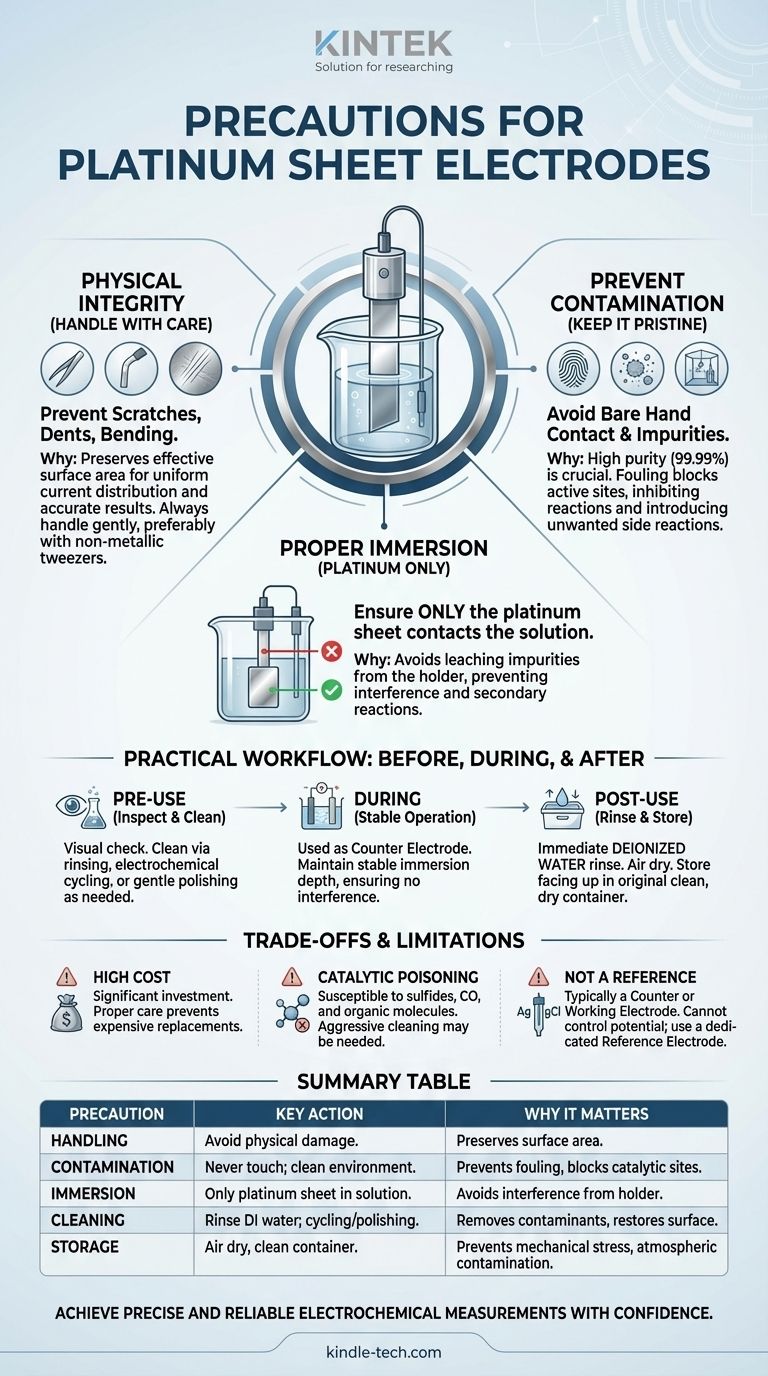
To properly use a platinum sheet electrode, you must prioritize three critical areas. First, handle it with extreme care to prevent physical damage like scratches or bending. Second, maintain a pristine surface by preventing contact with contaminants and cleaning it rigorously. Finally, ensure that only the platinum sheet itself, and no other part of the electrode assembly, ever touches the electrolyte solution.
The exceptional cost and sensitivity of a platinum electrode mean that proper care is not just about preventing damage. Meticulous handling and cleaning are fundamental prerequisites for generating accurate, reproducible, and trustworthy experimental data.

The Core Principles of Electrode Integrity
A platinum electrode's performance is directly tied to the condition of its surface. Understanding the "why" behind each precaution is key to mastering its use.
Preserving Mechanical Integrity
Platinum is a relatively soft metal. Scratches, dents, or deformations alter the electrode's effective surface area, which is a critical parameter in most electrochemical calculations.
A damaged surface leads to non-uniform current distribution, compromising the quality and interpretability of your results. Always handle the electrode gently, preferably with non-metallic tweezers if necessary.
Preventing Surface Contamination
The high purity of the platinum (often 99.99%) is what ensures its predictable catalytic behavior. Contamination, even from fingerprints or organic vapors, can "foul" or "poison" the electrode surface.
This fouling can block active sites, inhibit desired reactions, or introduce unwanted side reactions, invalidating your measurements. Never touch the platinum sheet with your bare hands and work in a clean environment.
Ensuring Proper Immersion
It is imperative that only the platinum sheet makes contact with your sample solution.
If other parts of the electrode holder (often made of glass or PEEK) are submerged, they can leach impurities or, worse, create a secondary, uncontrolled electrochemical cell that interferes with your primary measurement.
Understanding Its Chemical Environment
Platinum is exceptionally stable and resistant to corrosion in most strong acids and bases, making it a robust choice for a wide range of experiments.
However, be cautious in strongly reducing environments, as some compounds can interact with and degrade the platinum surface over time.
Practical Workflow: Before, During, and After Use
A consistent workflow is the best way to protect your electrode and ensure reliable data collection.
Pre-Experiment Inspection and Cleaning
Before every use, visually inspect the electrode. The surface should be smooth, bright, and uniform. If it appears dull or discolored, it requires cleaning.
Depending on the nature of the previous experiment, cleaning may involve rinsing with deionized water, electrochemical cycling in a cleaning solution (like dilute sulfuric acid), or gentle polishing with a specialized alumina slurry.
During the Experiment
The platinum sheet is most often used as a counter (or auxiliary) electrode. Its role is to complete the circuit and balance the charge from the reaction occurring at the working electrode, without interfering with it.
Keep the electrode stable and ensure its immersion depth does not change during the measurement.
Post-Experiment Rinsing and Storage
Immediately after the experiment concludes, rinse the electrode thoroughly with deionized water to remove all traces of electrolyte.
Allow it to air dry completely. Store it in its original container or a dedicated, clean, dry box with the platinum sheet facing upwards to prevent mechanical stress and atmospheric contamination.
Understanding the Trade-offs and Limitations
While platinum is an exceptional material, it is not without its challenges. Being aware of them is crucial for effective use.
The High Cost Factor
Platinum is a precious metal, and electrodes are a significant laboratory investment. The high cost underscores the importance of all other precautions, as replacing a damaged electrode is expensive.
Susceptibility to Catalytic Poisoning
While chemically robust, platinum's catalytic surface can be "poisoned" by specific substances like sulfides, carbon monoxide, or certain organic molecules. These compounds can adsorb strongly to the surface, deactivating it for the intended reaction.
If you suspect poisoning, a more aggressive cleaning or polishing procedure is required to restore the electrode's performance.
It Is Not a Reference Electrode
A common point of confusion for beginners is the different types of electrodes. A platinum sheet is typically a counter or working electrode. It does not have a stable, constant potential and cannot be used to measure or control the potential of your system.
For accurate potential control, you must use a separate, dedicated reference electrode, such as a Saturated Calomel Electrode (SCE) or a Silver/Silver Chloride (Ag/AgCl) electrode.
Making the Right Choice for Your Goal
Your experimental objective dictates which aspect of electrode care to emphasize.
- If your primary focus is high-precision quantitative analysis: Your priority is maintaining a perfectly clean and known surface area through rigorous, repeatable cleaning protocols before every experiment.
- If your primary focus is long-term use in diverse chemical systems: Leverage platinum's chemical resistance but be meticulous about post-experiment cleaning and storage to prevent cross-contamination and gradual surface degradation.
- If your primary focus is managing laboratory costs and throughput: Treat proper handling and storage procedures as a direct investment that prevents costly replacements and minimizes time lost to troubleshooting unreliable data.
Ultimately, treating your platinum electrode with meticulous care is the most direct path to producing data you can trust.
Summary Table:
| Precaution Category | Key Action | Why It Matters |
|---|---|---|
| Handling | Avoid scratches, dents, and bending. | Preserves surface area for accurate current distribution. |
| Contamination | Never touch with bare hands; work in a clean environment. | Prevents surface fouling that blocks catalytic sites. |
| Immersion | Ensure only the platinum sheet contacts the solution. | Avoids interference from other materials in the electrode assembly. |
| Cleaning | Rinse with deionized water; use electrochemical cycling or polishing. | Removes contaminants and restores a pristine, active surface. |
| Storage | Air dry completely and store in a clean, dry container. | Prevents mechanical stress and atmospheric contamination. |
Achieve precise and reliable electrochemical measurements with confidence. Proper electrode care is fundamental to data integrity. KINTEK specializes in high-quality lab equipment and consumables, including robust electrochemical cells and accessories designed to support your research.
Let our experts help you optimize your workflow and protect your investment. Contact our team today to discuss your specific laboratory needs and ensure your platinum electrodes deliver their best performance, experiment after experiment.
Visual Guide
Related Products
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Rotating Platinum Disk Electrode for Electrochemical Applications
- Gold Electrochemical Sheet Electrode Gold Electrode
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- What are the performance characteristics of platinum sheet electrodes? Unlock Superior Electrochemical Performance
- What are the specifications of the Platinum-Titanium Functional Electrode? Maximize Electrochemical Performance
- What are the key performance characteristics and applications of platinum sheets? Unmatched Reliability for Demanding Applications
- What is the most critical guideline for immersing a platinum sheet electrode in an electrolyte? Ensure Accurate Electrochemical Measurements
- How should a platinum sheet electrode be pretreated before use? Ensure Accurate Electrochemical Measurements



















