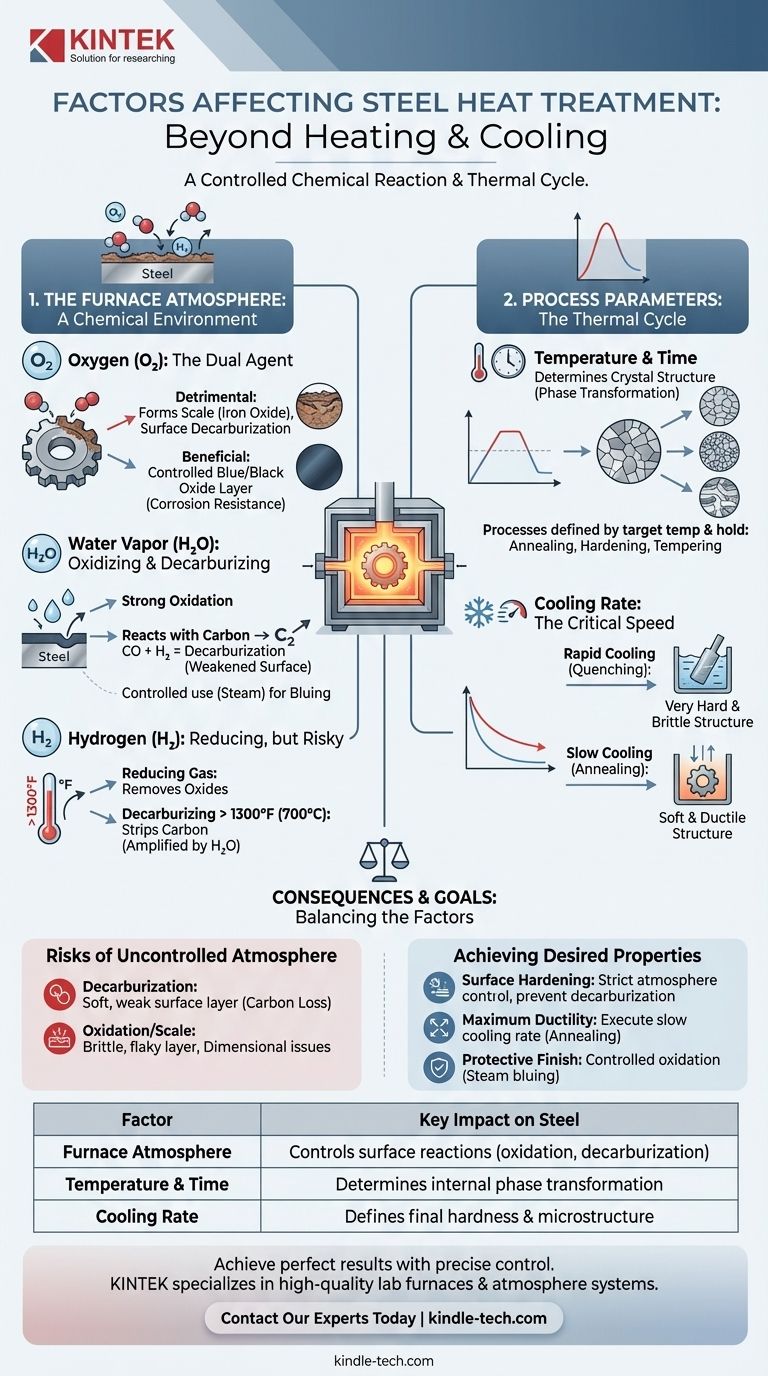
At its core, the success of any steel heat treatment is determined by two primary categories of factors: the specific thermal cycle (heating temperature, time, and cooling rate) and the chemical composition of the furnace atmosphere. The atmosphere, consisting of gases like oxygen, hydrogen, and water vapor, actively reacts with the steel's surface, fundamentally altering its properties.
Heat treatment is not merely a process of heating and cooling; it is a controlled chemical reaction. The gases within the furnace are active ingredients, not just an inert environment, and managing their effects is the key to achieving desired material properties while preventing defects.

The Critical Role of the Furnace Atmosphere
The environment surrounding the steel during heating is chemically reactive and has a direct, significant impact on the final product. Understanding the role of each gas is essential for process control.
The Duality of Oxygen
Oxygen is a highly reactive gas that can be both beneficial and detrimental. Its primary effect is reacting with the iron in steel to produce iron oxide, commonly known as scale.
This scale formation can be undesirable, altering part dimensions and surface finish. Furthermore, oxygen can cause surface decarburization by reacting with and removing carbon from the steel, resulting in a softer surface layer.
However, in some processes, oxygen is used intentionally. Controlled surface oxidation, often achieved with steam, can create a protective and aesthetically pleasing blue or black oxide layer that improves corrosion resistance.
The Impact of Water Vapor
Water vapor (H₂O) is a powerful oxidizing agent at heat treatment temperatures. It readily oxidizes the iron on the steel's surface.
Crucially, it also reacts with the carbon in the steel, forming carbon monoxide (CO) and hydrogen (H₂). This reaction is a significant cause of decarburization, which weakens the surface.
As with oxygen, this effect can be harnessed. Steam is often used as a bluing agent, intentionally creating a thin, controlled oxide layer on components like motor laminations.
Hydrogen's Reducing and Decarburizing Effects
Hydrogen is primarily a reducing gas, meaning it will react with and remove oxides. For instance, it can reduce iron oxide back to iron.
However, at temperatures above approximately 1300°F (700°C), hydrogen has a strong decarburizing effect on steel, stripping carbon from the surface. This effect is amplified by the presence of water vapor. Below this temperature, its decarburizing potential is negligible.
Understanding the Trade-offs and Inherent Risks
Controlling the furnace atmosphere is a balancing act. A failure to manage these chemical reactions leads to common and costly material defects.
The Pervasive Risk of Decarburization
Decarburization is the loss of carbon content from the surface of the steel. This creates a soft, weak outer layer on a component that was intended to be hard.
This is a major failure mode, as the component's wear resistance and fatigue strength depend on a hard, high-carbon surface. It is primarily caused by reactions with oxygen, water vapor, and high-temperature hydrogen.
The Problem of Oxidation and Scale
Scale (iron oxide) is the most visible byproduct of an uncontrolled atmosphere. This brittle, flaky layer can interfere with subsequent manufacturing steps like machining or coating.
Excessive scaling can also lead to a loss of material, causing the final part to be out of dimensional tolerance. Preventing unwanted scale requires minimizing the presence of oxidizing gases like oxygen and water vapor.
Process Parameters: Temperature, Time, and Cooling
Beyond the atmosphere, the physical parameters of the heat treatment cycle are the most fundamental factors of all.
Temperature and Time at Temperature
The temperature to which the steel is heated determines its crystal structure (phase). Holding the steel at that temperature for a specific duration allows this transformation to occur throughout the material.
Different processes, such as annealing (softening), hardening, and tempering (reducing brittleness), are all defined by unique target temperatures and hold times.
The Defining Role of Cooling Rate
The speed at which the steel is cooled from its treatment temperature is arguably the most critical factor in determining its final hardness and microstructure.
Quenching, or rapid cooling in a medium like water or oil, traps the steel in a very hard and brittle structure. In contrast, slow cooling, as seen in annealing, allows the structure to change into a soft and ductile form.
Making the Right Choice for Your Goal
Your specific objective dictates which factors you must prioritize and control most carefully.
- If your primary focus is surface hardening: You must strictly control the furnace atmosphere to prevent decarburization and, in processes like carburizing, actively use the atmosphere to add carbon to the surface.
- If your primary focus is achieving maximum ductility (softening): Your attention should be on executing the correct slow cooling rate from the annealing temperature.
- If your primary focus is creating a protective surface finish: You must intentionally introduce an oxidizing agent like steam at a precise temperature to grow a controlled oxide layer.
Mastering heat treatment is to master the controlled manipulation of steel's chemistry and structure through thermal energy and atmospheric reactions.
Summary Table:
| Factor | Key Impact on Steel |
|---|---|
| Furnace Atmosphere | Controls surface reactions (oxidation, decarburization) |
| Temperature & Time | Determines the steel's internal phase transformation |
| Cooling Rate | Defines final hardness and microstructure (e.g., quenching vs. annealing) |
Achieve perfect heat treatment results every time. The right lab equipment is critical for precise control over furnace atmosphere and thermal cycles. KINTEK specializes in high-quality lab furnaces, atmosphere control systems, and consumables designed to meet the exacting needs of metallurgy and materials testing laboratories.
Contact our experts today via our Contact Form to discuss how our solutions can help you prevent decarburization, control scaling, and consistently achieve your desired material properties.
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- What gases are used in inert atmospheres? Choose the Right Gas for Non-Reactive Environments
- What is an inert atmosphere heat treatment? Protect Your Metals from Oxidation & Decarburization
- What provides an inert atmosphere? Achieve Safety and Purity with Nitrogen, Argon, or CO2
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab



















