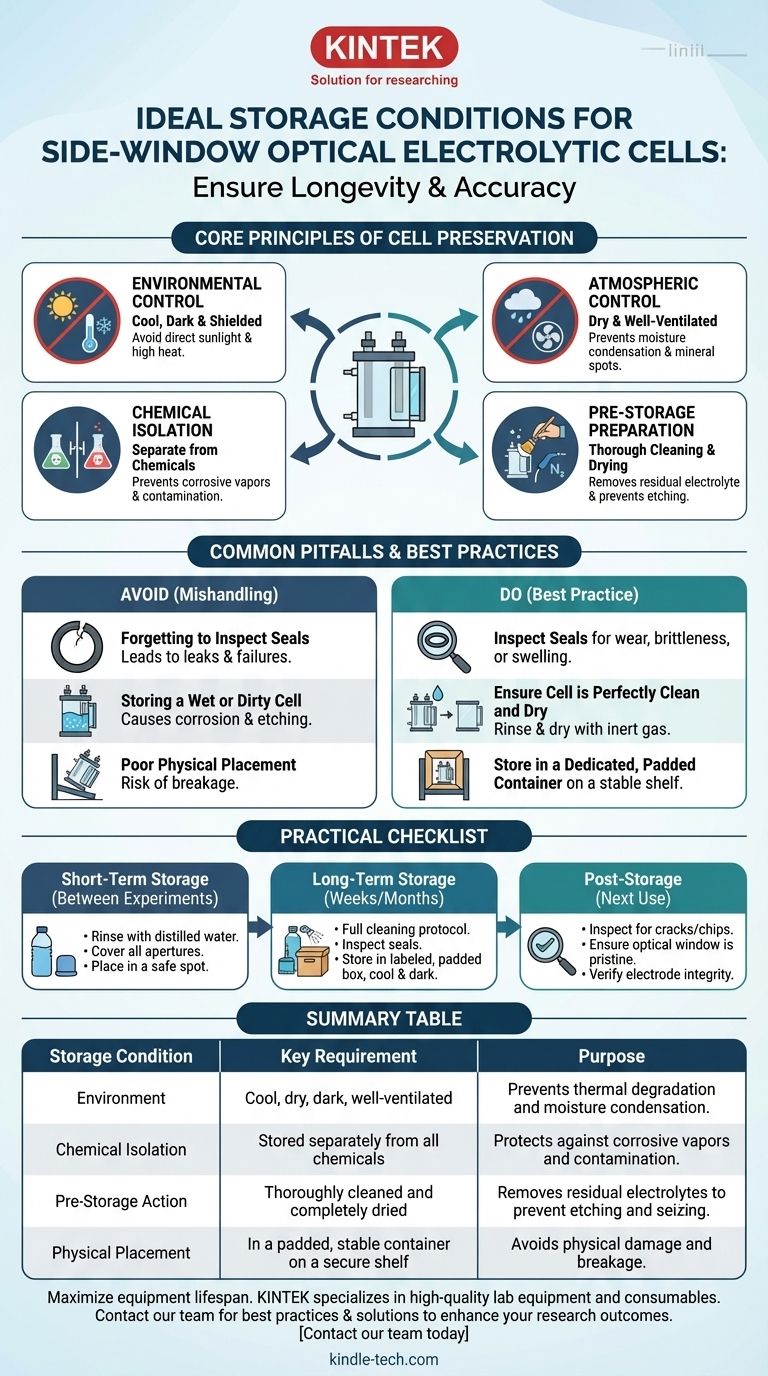
To ensure the longevity and accuracy of your side-window optical electrolytic cell, it must be stored in a dry, cool, and well-ventilated location. This environment must be shielded from direct sunlight and high temperatures. Crucially, the cell should be physically isolated from other laboratory chemicals to prevent material degradation and surface contamination.
The primary goal of proper storage is not merely to prevent breakage; it is a critical procedural step to preserve the cell's physical integrity and optical clarity, directly ensuring the accuracy and reproducibility of your future experiments.

The Core Principles of Cell Preservation
Properly storing your electrolytic cell involves creating a stable and inert environment. The key is to mitigate the four primary risks: thermal degradation, chemical contamination, humidity damage, and physical stress.
Environmental Control: Temperature and Light
High temperatures and direct UV exposure from sunlight can degrade the materials of the cell over time. This is especially true for any PTFE components, gaskets, or O-rings, which can become brittle or lose their sealing capability.
Atmospheric Control: Ventilation and Humidity
A dry, ventilated space prevents moisture from condensing on the cell's surfaces, particularly the optical window. Condensation can leave mineral spots upon evaporation that interfere with spectroscopic measurements and are difficult to remove. Proper ventilation also disperses any ambient chemical vapors in the lab that could otherwise settle on and contaminate the cell.
Chemical Isolation: Preventing Contamination
Storing the cell separately from other chemicals is paramount. Corrosive vapors, even in trace amounts, can etch the glass or degrade seals over long periods. This isolation prevents accidental reactions that could permanently damage the cell's sensitive optical and electrode surfaces.
Pre-Storage Preparation: The Importance of Cleaning
A cell must be thoroughly cleaned before it is put into storage. Any residual electrolyte left to dry can crystallize, potentially etching the glass window or seizing threaded components.
The standard cleaning protocol involves rinsing with distilled water, followed by soaking or rinsing with an appropriate organic solvent (like ethanol), and finally drying completely with a stream of inert gas such as nitrogen.
Common Pitfalls and Best Practices
Avoiding common mistakes during storage is just as important as following the correct procedure. Mishandling is often the root cause of premature cell failure.
Forgetting to Inspect Seals
The O-rings and seals that ensure the cell is leak-proof are its most vulnerable parts. Always inspect them for signs of wear, brittleness, or chemical swelling before storage. Storing a cell with a compromised seal can lead to future leaks and failed experiments.
Storing a Wet or Dirty Cell
This is the most frequent and damaging mistake. As mentioned, dried electrolyte salts are abrasive and corrosive. Ensure the cell is not just clean but perfectly dry before storing it to prevent long-term degradation.
Poor Physical Placement
Because many cells are made of glass, they are inherently fragile. Store the cell in a dedicated, labeled container, preferably with foam padding. It should be placed on a stable shelf where it is not at risk of being knocked over or having heavier items placed on top of it.
A Practical Checklist for Your Cell
Use this simple guide to determine the correct procedure based on your timeline and needs.
- If your goal is short-term storage (between experiments): Thoroughly rinse the cell with distilled water, cover all apertures to prevent dust from entering, and place it in a safe, designated spot away from traffic.
- If your goal is long-term storage (weeks or months): Perform a full cleaning protocol (water, solvent, nitrogen dry), inspect all seals, and store the cell in a labeled, padded box in a cool, dark, and dry cabinet away from all other chemicals.
- If you are preparing for the next use (post-storage): Carefully inspect the cell body for any new cracks or chips, ensure the optical window is pristine and transparent, and verify that all electrode interfaces are clean and intact.
Proper maintenance and storage are not chores; they are fundamental to generating reliable and high-quality data.
Summary Table:
| Storage Condition | Key Requirement | Purpose |
|---|---|---|
| Environment | Cool, dry, dark, and well-ventilated | Prevents thermal degradation and moisture condensation |
| Chemical Isolation | Stored separately from all other lab chemicals | Protects against corrosive vapors and surface contamination |
| Pre-Storage Action | Thoroughly cleaned and completely dried | Removes residual electrolytes to prevent etching and seizing |
| Physical Placement | In a padded, stable container on a secure shelf | Avoids physical damage and breakage |
Maximize the lifespan and accuracy of your laboratory equipment. Proper storage is just one part of a comprehensive maintenance strategy. KINTEK specializes in high-quality lab equipment and consumables, including optical electrolytic cells and the cleaning solvents needed to maintain them. Our experts can help you establish best practices to protect your investment and ensure data integrity.
Contact our team today to discuss your specific laboratory needs and discover how our products and support can enhance your research outcomes.
Visual Guide
Related Products
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results
- Why are stacked and rotated stainless steel wire meshes used in the cathode design of photoelectrochemical reactors?
- What is an electrolysis cell also known as? Understanding Electrolytic vs. Galvanic Cells
- What are the advantages of electrodeposition? Achieve Precision Coating for Complex Parts
- How should an H-type electrolytic cell be cleaned after use? A Step-by-Step Guide for Reliable Results
- Why are high-density graphite or metal baskets necessary for actinide oxide reduction? Key Roles in Direct Electrolysis
- What are the requirements for SO2 Depolarized Electrolyzer membranes? Optimize Performance in Hybrid Sulfur Cycles
- How should the H-type electrolytic cell be stored when not in use? Expert Storage & Maintenance Guide



















