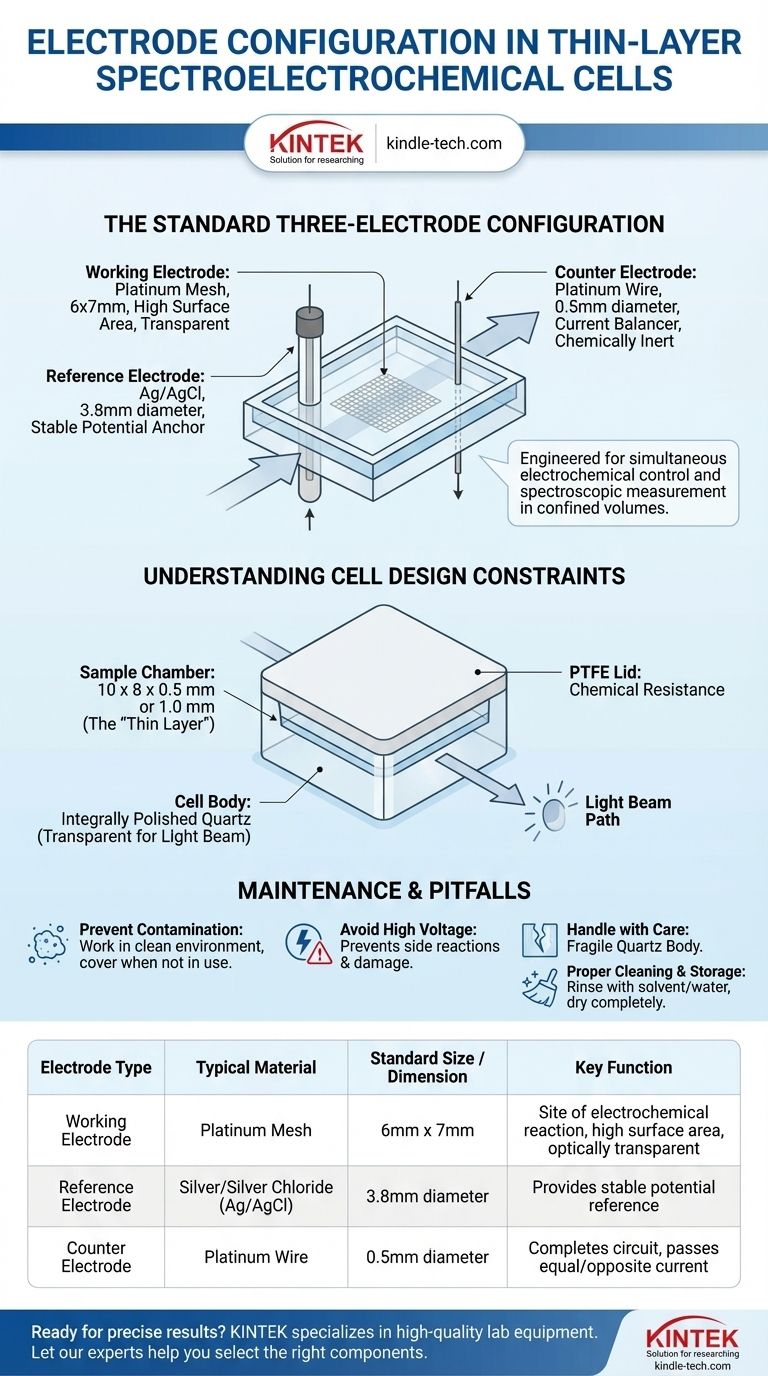
For a standard thin-layer spectroelectrochemical cell, the typical configuration involves a three-electrode system designed to fit within a small, optically transparent cuvette. This setup consists of a silver chloride reference electrode (3.8mm diameter), a platinum wire counter electrode (0.5mm diameter), and a platinum mesh working electrode with dimensions of 6x7mm.
The specific types and dimensions of these electrodes are not arbitrary; they are engineered to function precisely within the confined, thin-layer volume of the cell, enabling simultaneous electrochemical control and spectroscopic measurement of the analyte.

The Standard Three-Electrode Configuration
Understanding the role of each electrode is fundamental to performing successful spectroelectrochemical experiments. Each component is chosen for its specific properties and its ability to function within the cell's physical constraints.
The Working Electrode: The Site of Reaction
The working electrode is where the electrochemical reaction of interest takes place. A platinum mesh (6x7mm) is typically used for this purpose.
The mesh design provides a high surface area for the reaction while remaining sufficiently transparent for a light beam to pass through, which is essential for simultaneous spectroscopic analysis.
The Reference Electrode: The Stable Potential Anchor
The reference electrode provides a stable, constant potential against which the potential of the working electrode is measured and controlled.
A silver/silver chloride (Ag/AgCl) electrode with a diameter of 3.8mm is a common choice due to its stability in the aqueous and non-aqueous systems for which these cells are designed.
The Counter Electrode: The Current Balancer
The counter electrode, also known as the auxiliary electrode, completes the electrical circuit. It passes a current equal and opposite to that flowing at the working electrode.
A thin platinum wire (0.5mm diameter) is used because it is chemically inert and will not interfere with the primary reaction being studied at the working electrode.
Understanding the Cell's Design Constraints
The electrode dimensions are dictated by the physical design of the cell itself, which is optimized for thin-layer electrochemistry.
The "Thin-Layer" Dimension
The core of the design is the sample chamber, which has precise slit dimensions of either 10 x 8 x 0.5 mm or 10 x 8 x 1.0 mm. This narrow gap creates the "thin layer" of electrolyte.
This design ensures that the entire sample volume can be electrolyzed quickly and uniformly, a key requirement for many spectroelectrochemical techniques.
Material and Optical Properties
The cell body is made from integrally polished quartz, which is transparent on all four sides. This allows a spectrophotometer's light beam to pass through the cell without obstruction.
The lid is typically made of polytetrafluoroethylene (PTFE), a material known for its excellent chemical resistance, which prevents contamination and reaction with a wide range of solvents and electrolytes.
Common Pitfalls and Maintenance Protocols
Proper handling and maintenance are critical for ensuring data accuracy and extending the life of the cell and its components.
Preventing Contamination and Damage
Contaminants like dust can significantly affect results in such a small volume. Always work in a clean environment and keep the cell covered when not in use.
Avoid applying excessively high voltage, which can cause unwanted side reactions, electrolyte decomposition, or permanent damage to the electrodes.
Proper Cleaning and Storage
After an experiment, immediately turn off the power source before disconnecting the cell. Empty the electrolyte and rinse the cell and electrodes multiple times with distilled water or an appropriate solvent.
Ensure all components are completely dry before storing them in a clean, dry environment. For long-term storage, remove the electrolyte and seal the cell to protect it from moisture and dust.
Fragility of the Quartz Body
The quartz cell body is fragile and must be handled with care at all times. A single drop or impact can easily break it, rendering the entire apparatus unusable.
Making the Right Choice for Your Goal
Your experimental priorities will guide how you handle and maintain your spectroelectrochemical cell.
- If your primary focus is obtaining reliable data: Meticulous cleaning and adherence to operational protocols are paramount to prevent contamination and ensure reproducible results.
- If your primary focus is equipment longevity: Emphasize careful handling of the fragile quartz cell and follow the correct post-experiment drying and storage procedures for the electrodes.
- If your primary focus is experimental safety: Always use appropriate protective gear when handling corrosive electrolytes and double-check electrode polarity to prevent electrical hazards.
Mastering the function and care of these integrated components is the key to successful spectroelectrochemical analysis.
Summary Table:
| Electrode Type | Typical Material | Standard Size / Dimension | Key Function |
|---|---|---|---|
| Working Electrode | Platinum Mesh | 6mm x 7mm | Site of electrochemical reaction, high surface area, optically transparent |
| Reference Electrode | Silver/Silver Chloride (Ag/AgCl) | 3.8mm diameter | Provides stable potential reference |
| Counter Electrode | Platinum Wire | 0.5mm diameter | Completes circuit, passes equal/opposite current |
Ready to configure your spectroelectrochemical setup for precise, reliable results?
KINTEK specializes in high-quality lab equipment and consumables, including the precise electrodes and durable quartz cells essential for successful thin-layer spectroelectrochemical analysis. Our products are designed to meet the exacting standards of laboratory research, ensuring accurate data and long-lasting performance.
Let our experts help you select the right components for your specific application. Contact KINTEK today to discuss your laboratory needs and discover how our solutions can enhance your research efficiency and data quality.
Visual Guide
Related Products
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- What are the dimensions for thin-layer spectroelectrochemical cells? Optimize Your Lab's Optical Path Length
- What general precautions should be taken when using a thin-layer spectroelectrochemical cell? Ensure Accurate Results and Equipment Safety
- What is the correct post-experiment procedure for a thin-layer spectroelectrochemical cell? A Step-by-Step Guide for Lab Safety and Accuracy
- What preparation steps are required before initiating an experiment with the thin-layer spectroelectrochemical cell?
- For what types of systems, temperature ranges, and sealing configurations is the thin-layer spectroelectrochemical cell designed? Ideal for Aqueous and Non-Aqueous Analysis



















