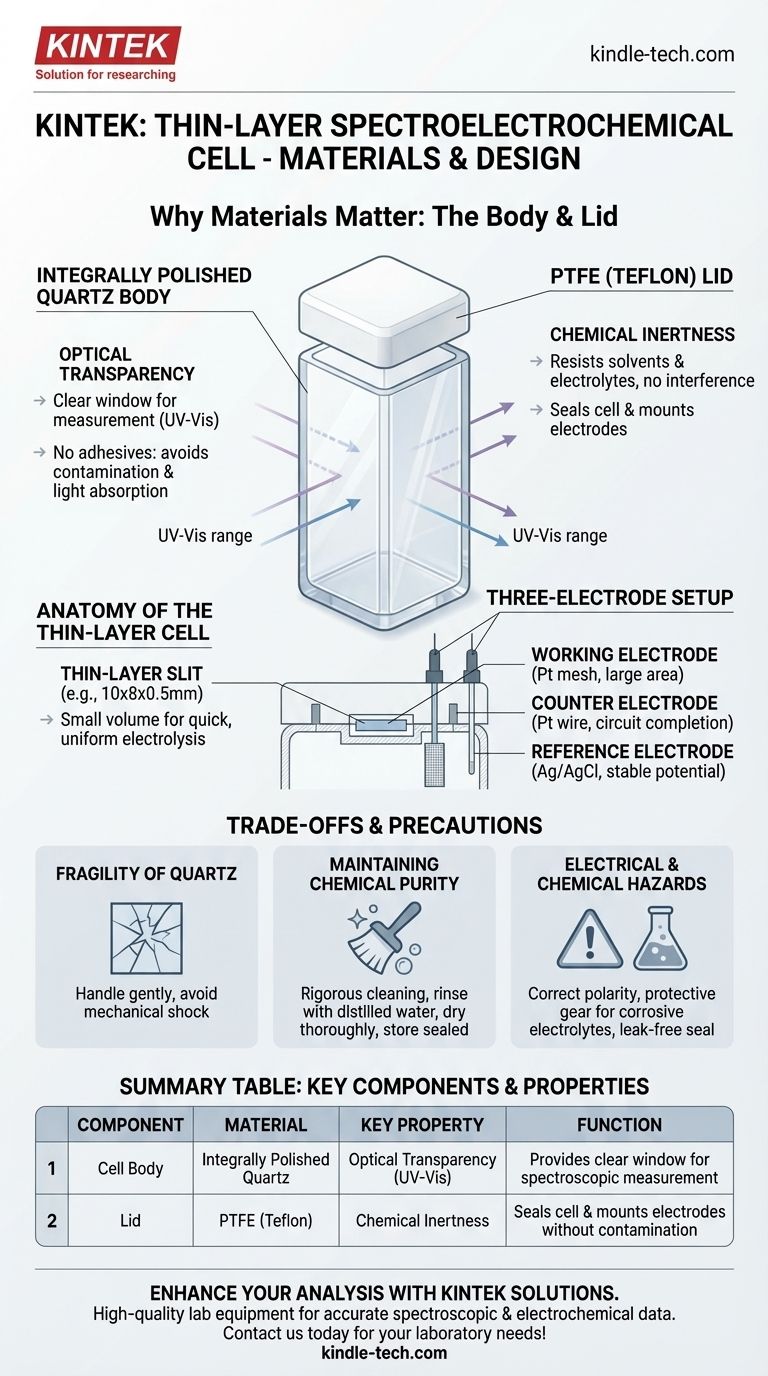
To be effective, a thin-layer spectroelectrochemical cell body is constructed from integrally polished quartz, while its lid is made from polytetrafluoroethylene (PTFE). This specific combination is essential for the cell's dual function. The quartz provides unparalleled optical transparency for spectroscopic analysis, and the PTFE delivers the chemical inertness required to contain the electrochemical reaction without interference.
The choice of quartz and PTFE is not arbitrary; it is a direct solution to the core requirements of spectroelectrochemistry. Quartz provides the necessary optical window for spectroscopic measurement, while PTFE ensures a chemically inert seal, protecting the sensitive electrochemical reaction within.
Why These Materials Are Critical
The materials used in a spectroelectrochemical cell directly enable its ability to simultaneously probe a sample with both light and electricity. Their properties are chosen to maximize data quality and minimize experimental error.
The Quartz Body: A Clear Optical Window
The body of the cell is essentially a specialized cuvette. It is made from integrally polished quartz because of its superior optical properties.
This material is transparent across a broad range of the electromagnetic spectrum, especially in the UV-Vis region, which is crucial for most "spectro-" experiments. The design specifies that it is transparent on all four sides, allowing for flexibility in how the light source and detector are positioned.
Furthermore, the cell is assembled without adhesives. This is a critical detail, as adhesives could leach into the solution, contaminating the experiment, or absorb light, interfering with the spectroscopic measurement.
The PTFE Lid: An Inert and Functional Seal
The lid is made from polytetrafluoroethylene (PTFE), a fluoropolymer widely known by the trade name Teflon.
PTFE is chosen for its exceptional chemical inertness. It resists degradation from the vast majority of solvents and electrolytes used in electrochemistry, ensuring that the lid itself does not become part of the reaction.
The lid serves as the mounting point for the electrodes. Its material must be easily and precisely machined to accommodate the reference, counter, and working electrodes, ensuring they are correctly positioned within the cell's thin layer.
Anatomy of the Thin-Layer Cell
Understanding the materials is the first step. Understanding how they fit into the cell's overall design reveals why it is such a powerful analytical tool.
The Cell Body and Slit
The cell typically has a standard square footprint (e.g., 12x12mm) to fit into spectrophotometer sample holders.
The key feature is the internal thin-layer slit (e.g., 10x8x0.5mm). This extremely small, defined volume ensures that the entire solution in the light path can be electrolyzed quickly and uniformly, which is the foundational principle of thin-layer electrochemistry.
The Electrode Configuration
A standard three-electrode setup is inserted through the PTFE lid.
- Working Electrode: A platinum mesh is common, providing a large surface area for the reaction.
- Counter Electrode: A platinum wire serves to complete the electrical circuit.
- Reference Electrode: A silver chloride electrode provides a stable potential against which the working electrode's potential is controlled.
Understanding the Trade-offs and Handling Precautions
While highly effective, the materials and design of the cell demand careful handling to ensure accurate results and a long lifespan.
The Fragility of Quartz
The primary drawback of the quartz body is its fragility. It is brittle and must be handled with care to prevent chipping or breakage, which would render the cell useless. Always avoid mechanical shock and handle the cell gently.
Maintaining Chemical Purity
Contaminants are the enemy of both spectroscopy and electrochemistry. Dust or chemical residues can absorb light or participate in unwanted side reactions.
Therefore, rigorous cleaning with appropriate solvents, followed by rinsing with distilled water and thorough drying, is mandatory before and after each use. Store the dry, clean cell in a sealed container to protect it from dust.
Preventing Electrical and Chemical Hazards
Always connect the electrodes with the correct polarity. Reversing the connections can damage the electrodes or produce invalid results.
When using corrosive electrolytes, wear appropriate protective gear. Ensure the PTFE lid creates a good seal to prevent leaks, protecting both the user and the surrounding equipment.
Making the Right Choice for Your Experiment
Your experimental goal dictates which aspects of cell maintenance and setup require the most attention.
- If your primary focus is accurate spectroscopic data: Ensure the quartz body is impeccably clean, as any smudge or residue on the optical faces will directly impact your results.
- If your primary focus is reliable electrochemical results: Prioritize correct electrode placement, a pure deoxygenated electrolyte, and a leak-free seal to maintain the integrity of the reaction environment.
- If your primary focus is long-term usability and safety: Adhere strictly to the protocols for cleaning, drying, and storage to protect the fragile quartz and prevent electrode corrosion over time.
By understanding the function and limitations of these materials, you can ensure the integrity of your experiment and the longevity of this powerful analytical tool.
Summary Table:
| Component | Material | Key Property | Function |
|---|---|---|---|
| Cell Body | Integrally Polished Quartz | Optical Transparency (UV-Vis) | Provides a clear window for spectroscopic measurement |
| Lid | PTFE (Teflon) | Chemical Inertness | Seals the cell and mounts electrodes without contamination |
Ready to enhance your spectroelectrochemical analysis? KINTEK specializes in high-quality lab equipment and consumables, including precision cells designed for superior performance. Our expertise ensures you get the right tools for accurate spectroscopic and electrochemical data. Contact us today to discuss your laboratory needs and discover how our solutions can drive your research forward!
Visual Guide
Related Products
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the physical dimensions of the thin-layer spectroelectrochemical cell body and its slit? Key Specs for Your Lab
- What are the necessary preparation steps before using a thin-layer spectroelectrochemical cell? A Guide to Reliable Results
- What is the correct post-experiment procedure for a thin-layer spectroelectrochemical cell? A Step-by-Step Guide for Lab Safety and Accuracy
- Which electrode types are compatible with thin-layer spectroelectrochemical cells? Optimize Your Hardware Fit
- What preparation steps are required before initiating an experiment with the thin-layer spectroelectrochemical cell?



















