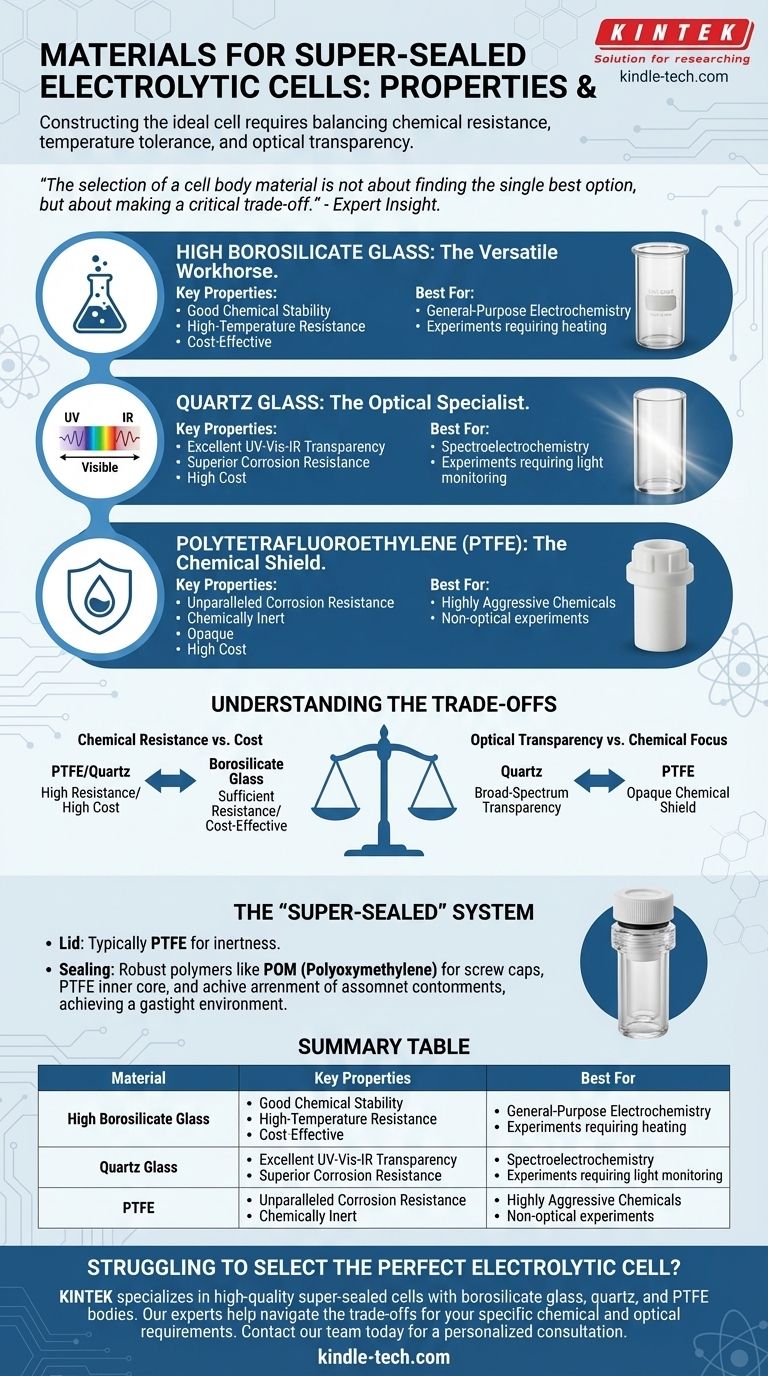
For a super-sealed electrolytic cell, the body is most commonly constructed from high borosilicate glass, quartz glass, or Polytetrafluoroethylene (PTFE). The choice depends on the specific demands of the experiment, balancing chemical resistance, temperature tolerance, and the need for optical transparency. Lids and sealing components are typically made from chemically inert materials like PTFE and Polyoxymethylene (POM) to ensure a gastight seal.
The selection of a cell body material is not about finding the single "best" option, but about making a critical trade-off. You must align the material's specific properties—such as chemical inertness or light transparency—with the precise requirements of your electrochemical experiment.

Core Body Materials and Their Properties
The main vessel of the cell, or the body, must contain the electrolyte and withstand the experimental conditions. The three primary materials used each offer a distinct set of advantages.
High Borosilicate Glass: The Versatile Workhorse
High borosilicate glass is a frequent choice for general-purpose electrolytic cells. Its popularity stems from a balanced profile of useful properties.
It offers very good chemical stability and is resistant to many common electrolytes. It is also known for its high-temperature resistance, making it suitable for experiments that require heating.
Quartz Glass: The Optical Specialist
When an experiment involves light, such as in spectroelectrochemistry, quartz glass is the superior material.
Its key feature is excellent light transmittance across the entire spectrum, from ultraviolet (UV) to visible and infrared (IR). It also provides excellent corrosion resistance, withstanding strong acids and weak bases. Its only significant chemical vulnerability is to hydrofluoric acid.
Polytetrafluoroethylene (PTFE): The Chemical Shield
PTFE, widely known by the brand name Teflon, is used when ultimate chemical inertness is the primary concern.
This material provides unparalleled corrosion resistance, making it the ideal choice for experiments involving highly aggressive chemical agents. However, unlike glass or quartz, PTFE is opaque, making it unsuitable for any optical measurements through the cell body.
Understanding the Trade-offs
Choosing the right material requires you to prioritize your experimental needs. A material that is perfect for one application can be entirely wrong for another.
Chemical Resistance vs. Cost
A PTFE or quartz glass body offers the highest degree of chemical inertness for aggressive media. However, these are premium materials and come at a higher cost.
High borosilicate glass provides sufficient resistance for a wide range of applications and is a more cost-effective solution for standard electrochemical work.
Optical Transparency vs. Chemical Focus
If your work requires monitoring reactions with UV-Vis or other forms of spectroscopy, the choice is clear. Quartz glass is essential for its broad-spectrum transparency.
If the experiment is purely electrochemical with no optical component, and you are using highly corrosive materials, an opaque PTFE body is often the safer and more durable option.
The "Super-Sealed" System
The term "super-sealed" refers to the entire assembly, not just the body. The cell body material is only part of the equation for achieving a truly inert, gastight environment.
The lid is almost always made of PTFE for its chemical inertness. The sealing mechanism itself often involves an external thread structure, using a PTFE inner core and robust polymers like POM (Polyoxymethylene) for the screw caps to apply firm, even pressure.
Making the Right Choice for Your Experiment
Your decision should be guided by the primary goal of your research.
- If your primary focus is general-purpose electrochemistry: High borosilicate glass offers the best balance of performance, thermal stability, and cost.
- If your primary focus is spectroelectrochemistry or requires UV transparency: Quartz glass is the only material that provides the necessary optical clarity across the spectrum.
- If your primary focus is working with highly aggressive chemicals: A cell body made of PTFE provides the ultimate chemical shield, provided you do not need optical access.
Ultimately, selecting the correct material is the first step in ensuring the integrity and success of your electrochemical data.
Summary Table:
| Material | Key Properties | Best For |
|---|---|---|
| High Borosilicate Glass | Good chemical stability, high-temperature resistance, cost-effective | General-purpose electrochemistry |
| Quartz Glass | Excellent UV-Vis-IR transparency, superior corrosion resistance | Spectroelectrochemistry, experiments requiring light |
| PTFE (Teflon) | Unparalleled chemical inertness, opaque | Highly aggressive chemicals, non-optical experiments |
Struggling to select the perfect electrolytic cell for your specific chemical and optical requirements?
KINTEK specializes in high-quality lab equipment, including a range of super-sealed electrolytic cells with bodies made from high borosilicate glass, quartz, and PTFE. Our experts can help you navigate the trade-offs between chemical resistance, optical clarity, and cost to ensure your experimental integrity and success.
Contact our team today for a personalized consultation and find the ideal cell for your research!
Visual Guide
Related Products
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What are the standard opening specifications for all-PTFE electrolytic cells? A Guide to Sealed vs. Non-Sealed Ports
- What precautions should be taken during the storage of an all-PTFE electrolytic cell? Prevent Permanent Deformation and Seal Failure
- What are the key material properties and structural features of an all-PTFE electrolytic cell? Achieve Unmatched Purity in Harsh Electrochemical Environments
- What inspection steps should be performed on an all-PTFE electrolytic cell before use? Ensure Reliable Results
- How should an all-PTFE electrolytic cell be handled to prevent mechanical damage? Protect Your Investment and Data Integrity



















