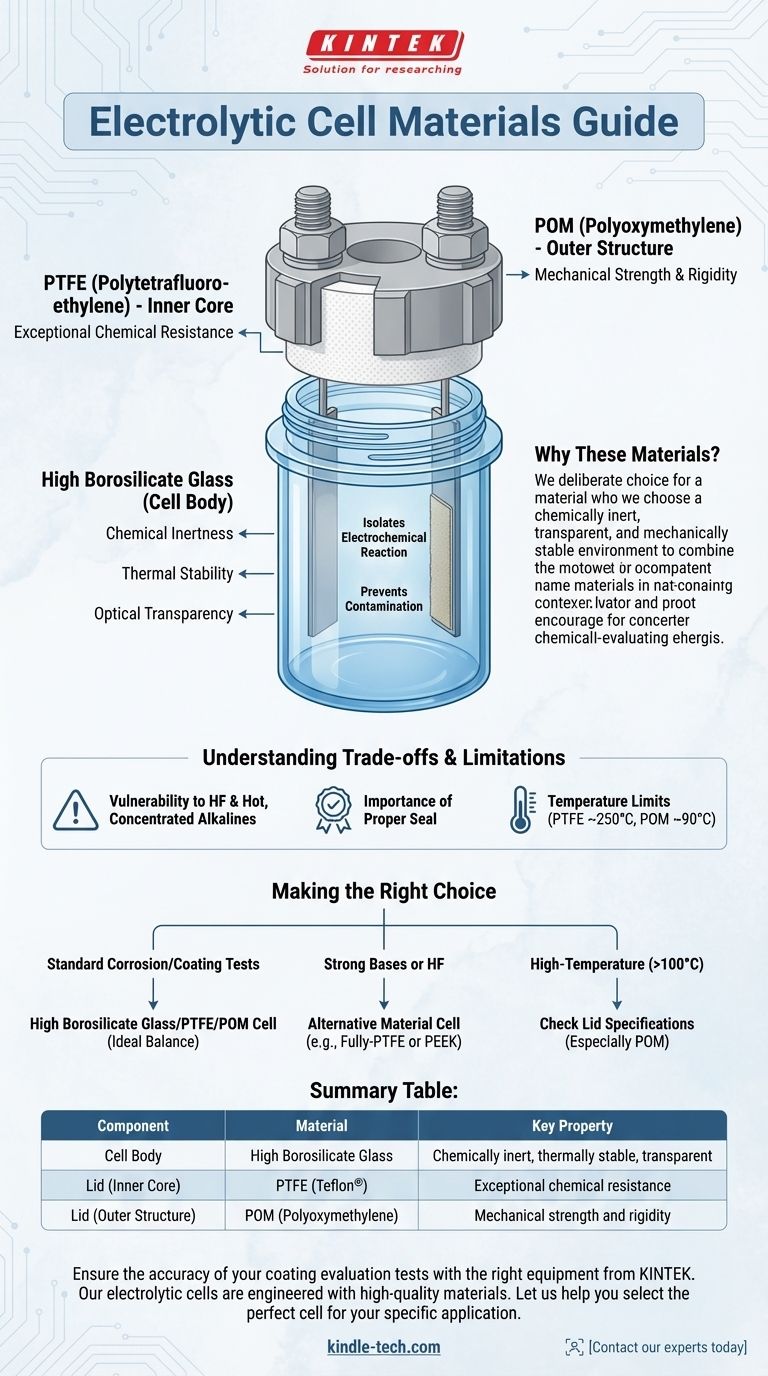
In a typical coating evaluation electrolytic cell, the body is constructed from high borosilicate glass. The lid is a composite structure where the inner core that contacts the solution is made from Polytetrafluoroethylene (PTFE), and the outer mechanical components for sealing are often made from Polyoxymethylene (POM).
The selection of these materials is not arbitrary; it is a deliberate engineering choice. The goal is to create a chemically inert, transparent, and mechanically stable environment that isolates the electrochemical reaction, ensuring that the measurement results are not skewed by contamination from the cell itself.

The Role of High Borosilicate Glass (The Cell Body)
The cell body acts as the primary container for the electrolyte and the electrodes. The choice of high borosilicate glass is critical for several reasons.
Chemical Inertness
High borosilicate glass is highly resistant to chemical attack from a wide range of acids, neutral solutions, and organic solvents. This inertness is crucial for preventing the glass itself from leaching ions into the electrolyte, which would contaminate the experiment and produce inaccurate data.
Thermal Stability
This type of glass has a very low coefficient of thermal expansion, making it highly resistant to thermal shock. This allows for experiments to be conducted at varying temperatures without risking cracks or failure of the cell.
Optical Transparency
Being transparent allows the researcher to visually monitor the experiment. You can observe the working electrode for coating delamination, bubble formation (e.g., hydrogen evolution), or color changes in the solution, providing valuable qualitative data alongside the electronic measurements.
Deconstructing the Multi-Material Lid
The lid is arguably more complex than the body because it must provide a tight seal while accommodating multiple electrodes and being completely non-reactive with the electrolyte.
PTFE (Polytetrafluoroethylene) - The Inner Core
The part of the lid that faces the inside of the cell and comes into direct contact with the electrolyte and its vapors is made of PTFE. This material, commonly known by the brand name Teflon®, has exceptional chemical resistance, even to highly aggressive acids and bases.
Using PTFE for the inner core ensures that nothing from the lid can dissolve or react with the test solution, maintaining the purity of the electrochemical system.
POM (Polyoxymethylene) - The Outer Structure
While PTFE is chemically robust, it is a relatively soft material. For the mechanical parts of the lid, such as the screw caps and nuts that create the seal, a more rigid and durable polymer is needed.
POM is a strong, stiff engineering thermoplastic that provides the mechanical stability required to securely fasten the lid and seal the electrodes. It is used for the external components that do not touch the electrolyte.
Understanding the Trade-offs and Limitations
While this material combination is excellent for general use, it is essential to be aware of its limitations to prevent failed experiments or equipment damage.
Vulnerability of Glass to Certain Chemicals
High borosilicate glass is not completely infallible. It can be etched and damaged by hydrofluoric acid (HF) and hot, concentrated alkaline solutions (e.g., sodium hydroxide). Using these electrolytes requires a different type of cell, often one made entirely of a resistant polymer like PTFE or PEEK.
The Importance of a Proper Seal
The effectiveness of the lid relies on the proper assembly of the PTFE core and the POM outer structure. An improperly tightened or misaligned lid can lead to electrolyte leakage or the ingress of atmospheric oxygen, which can critically interfere with many corrosion studies.
Temperature Limits of Polymers
While the glass body can handle high temperatures, the polymer components of the lid have a much lower operating limit. Both PTFE and POM have maximum service temperatures that should not be exceeded, typically around 250°C for PTFE and 90°C for POM under load.
Making the Right Choice for Your Experiment
Your experimental conditions dictate whether a standard glass/PTFE cell is appropriate.
- If your primary focus is standard corrosion or coating tests in neutral or acidic aqueous solutions: The standard high borosilicate glass cell with a PTFE/POM lid is the ideal choice for its balance of visibility, purity, and durability.
- If your primary focus is experiments involving strong bases or hydrofluoric acid: You must use a cell made from an alternative material, such as a fully-PTFE or PEEK vessel, to prevent chemical attack on the glass body.
- If your primary focus is high-temperature experiments above 100°C: Scrutinize the material specifications of the lid components (especially POM) to ensure they can withstand the target temperature without deforming.
Understanding the material composition of your electrolytic cell is the first step toward ensuring the accuracy and reliability of your electrochemical data.
Summary Table:
| Component | Material | Key Property |
|---|---|---|
| Cell Body | High Borosilicate Glass | Chemically inert, thermally stable, transparent |
| Lid (Inner Core) | PTFE (Teflon®) | Exceptional chemical resistance |
| Lid (Outer Structure) | POM (Polyoxymethylene) | Mechanical strength and rigidity |
Ensure the accuracy of your coating evaluation tests with the right equipment from KINTEK.
Our electrolytic cells are engineered with high-quality materials like borosilicate glass and PTFE to provide the chemically inert environment your experiments demand. Whether you're conducting standard corrosion tests or working with aggressive chemicals, KINTEK's lab equipment is designed for reliability and precision.
Let us help you select the perfect cell for your specific application. Contact our experts today to discuss your laboratory needs and discover how our solutions can enhance your research outcomes.
Visual Guide
Related Products
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Electrolytic Electrochemical Cell with Five-Port
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- How does a dual-chamber bio-electrochemical cell configuration assist in electrode characterization? Enhanced Isolation.
- What is the purpose of an electrolytic etching system for 310H stainless steel? Reveal Precise Microstructure Details
- Why is it important to avoid short-circuiting the electrodes in an electrolytic cell? Prevent Catastrophic Equipment Failure
- What should be observed during an experiment with the H-type electrolytic cell? Key Monitoring for Precise Results
- What are the primary functions of a specialized electrowinning cell? Optimize Gold Recovery and Purity
- What are the specifications of the openings on the electrolytic cell? A Guide to Port Sizes and Configurations
- What is the main difference between galvanic cell and electrolytic cell? A Clear Guide to Energy Conversion
- How does an electrochemical reaction system optimize titanium surfaces? Engineering Bioactive Dental Implants



















