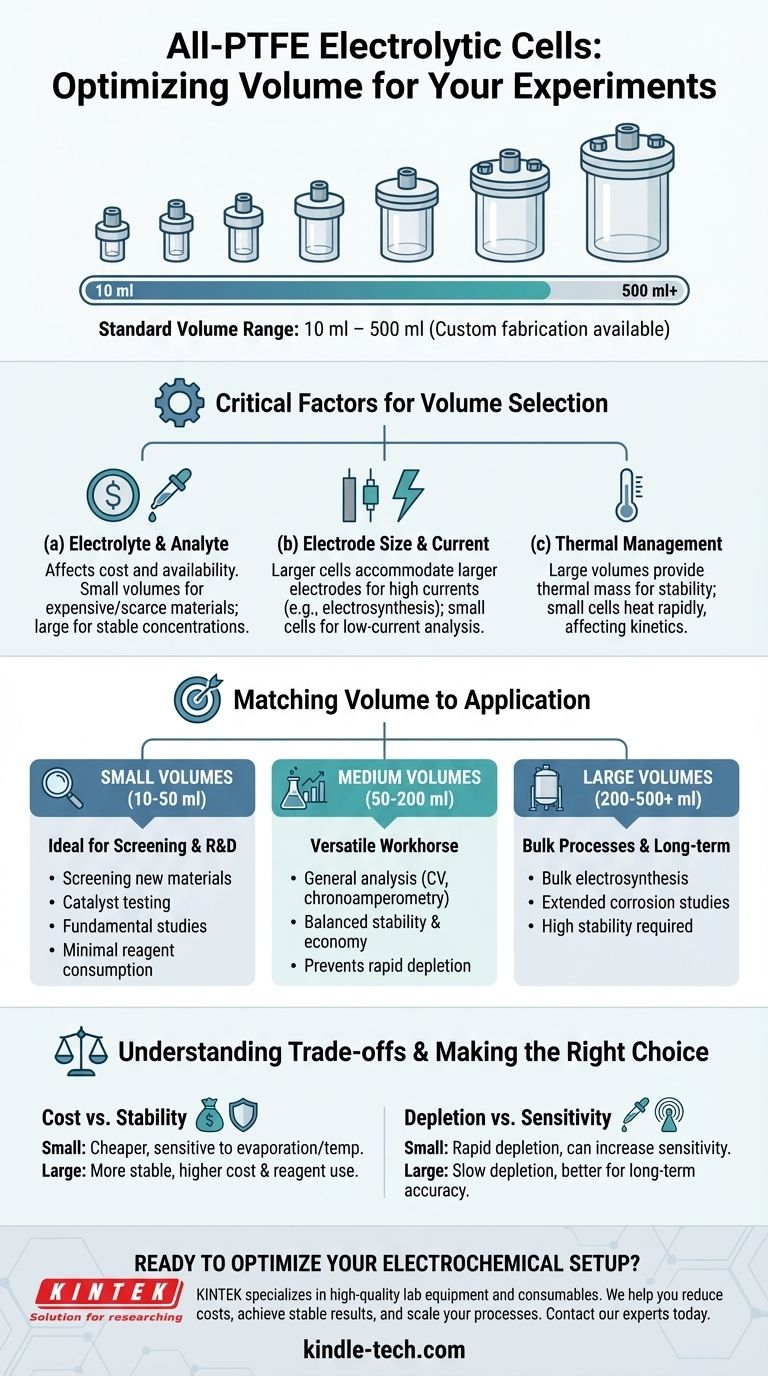
To be direct, all-PTFE electrolytic cells, which are standard for three-electrode systems, are typically available in volumes ranging from 10 ml to 500 ml. While this is a common commercial range, it's important to note that custom fabrication is often possible for specific experimental needs.
Choosing the right cell volume is more than a matter of convenience; it is a critical experimental decision that directly influences material costs, current requirements, and the stability of your electrochemical system.

Why Cell Volume Is a Critical Experimental Parameter
The volume of your cell is not just a container size. It dictates fundamental aspects of your experiment, from the amount of chemical reagent you use to the type of data you can reliably collect.
The Role of Electrolyte and Analyte
The most immediate factor is the cost and availability of your materials.
Small volumes are essential when working with expensive electrolytes, custom-synthesized catalysts, or scarce analytes. This minimizes waste and makes exploratory research feasible.
Conversely, larger volumes are necessary for experiments where you need to minimize concentration changes over time, such as in bulk electrolysis or long-duration tests.
Impact on Electrode Size and Current
Cell volume is intrinsically linked to the size of the electrodes it can accommodate.
A larger cell is required to house larger working and counter electrodes. This is crucial for applications that demand high currents, like preparative electrosynthesis, where the goal is to produce a measurable amount of product.
Smaller cells are perfectly suited for low-current analytical techniques where micro- or millimeter-scale electrodes are used for sensitive measurements.
Thermal Management
Electrochemical reactions can generate heat, and the cell's volume plays a key role in managing it.
Larger volumes of electrolyte provide greater thermal mass, meaning they can absorb more heat without a significant rise in temperature. This creates a more stable operating environment.
Small-volume cells can heat up very quickly, which can alter reaction kinetics and potentially degrade your sample or electrolyte if not properly controlled.
Matching Cell Volume to Your Application
Different electrochemical goals demand different cell configurations. Understanding this relationship is key to selecting the right equipment.
Small Volumes (10-50 ml): Ideal for Screening
These cells are the standard for initial research and development.
They are perfect for screening new materials, catalyst testing, and fundamental mechanistic studies where you need to perform many experiments with minimal reagent consumption.
Medium Volumes (50-200 ml): The Versatile Workhorse
This range represents a common balance for general-purpose research labs.
It provides enough volume to ensure that the analyte is not quickly depleted during standard analyses like cyclic voltammetry or chronoamperometry, while still being economical with materials.
Large Volumes (200-500+ ml): Suited for Bulk Processes
When the goal is production or long-term observation, a larger cell is non-negotiable.
These are essential for bulk electrosynthesis or extended corrosion studies on larger material samples, where stable conditions must be maintained for hours or even days.
Understanding the Trade-offs
Every choice in experimental design involves a compromise. Selecting a cell volume is a classic example of balancing competing priorities.
The Cost vs. Stability Dilemma
Smaller cells are cheaper to run but are far more sensitive to environmental changes. Evaporation can significantly alter concentrations, and they are more susceptible to temperature fluctuations.
Larger cells offer superior stability but come at a higher initial and running cost due to the greater volume of high-purity solvents and electrolytes required.
The Depletion vs. Sensitivity Problem
In a small cell, a reaction can rapidly deplete the analyte near the electrode surface, which can complicate the interpretation of results in long-term experiments.
However, for some sensing applications, this rapid change in a small volume can actually increase the sensitivity of the measurement, making it a desirable feature.
Making the Right Choice for Your Goal
Your specific research objective should be the ultimate guide in selecting an appropriate cell volume.
- If your primary focus is screening catalysts or working with costly materials: A smaller volume (10-50 ml) is the most efficient and economical choice.
- If your primary focus is general electrochemical characterization and analysis: A medium volume (50-200 ml) offers the best balance of experimental stability and resource management.
- If your primary focus is bulk electrosynthesis or long-term corrosion testing: A larger volume (200 ml and above) is necessary to accommodate the required scale and ensure stable conditions.
Ultimately, selecting the correct cell volume is a foundational step toward obtaining reliable and reproducible electrochemical data.
Summary Table:
| Application | Recommended Volume | Key Considerations |
|---|---|---|
| Screening & R&D | 10 - 50 ml | Minimizes reagent cost; ideal for expensive materials. |
| General Analysis | 50 - 200 ml | Balances stability and material economy for techniques like cyclic voltammetry. |
| Bulk Processes | 200 - 500+ ml | Essential for electrosynthesis and long-term corrosion studies requiring high stability. |
Ready to Optimize Your Electrochemical Setup?
Choosing the right PTFE electrolytic cell is crucial for the success and reproducibility of your experiments. KINTEK specializes in high-quality lab equipment and consumables, serving the precise needs of electrochemical researchers.
We provide reliable all-PTFE cells and expert guidance to help you:
- Reduce material costs by selecting the optimal cell volume for your specific reagents.
- Achieve stable results with equipment designed for thermal management and long-term experiments.
- Scale your processes from initial screening to bulk synthesis with confidence.
Let's discuss your project requirements. Contact our experts today to find the perfect electrolytic cell for your laboratory's needs.
Visual Guide
Related Products
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What materials are used for the body of a super-sealed electrolytic cell and what are their properties? Select the Right Material for Your Experiment
- What are the key material properties and structural features of an all-PTFE electrolytic cell? Achieve Unmatched Purity in Harsh Electrochemical Environments
- What is the precaution regarding temperature when using an all-PTFE electrolytic cell? Essential Thermal Safety Tips
- What are the standard opening specifications for all-PTFE electrolytic cells? A Guide to Sealed vs. Non-Sealed Ports
- How should an all-PTFE electrolytic cell be handled to prevent mechanical damage? Protect Your Investment and Data Integrity



















