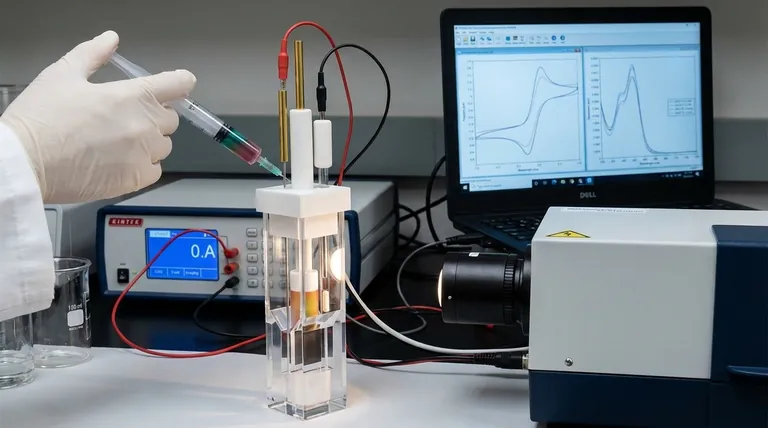
At its core, operating a thin-layer spectroelectrochemical cell involves a synchronized procedure. You must first securely connect the cell's electrodes to a potentiostat and align the cell within the spectrometer's light path. Next, carefully inject the electrolyte solution, set the desired electrochemical parameters (like potential or current), and then simultaneously initiate the electrochemical experiment and the spectroscopic data acquisition.
The fundamental challenge is not just performing an electrochemical experiment, but precisely correlating every change in the substance's optical properties (its spectrum) with a specific electrochemical event (its potential or current). Success depends on meticulous setup and synchronized data collection.
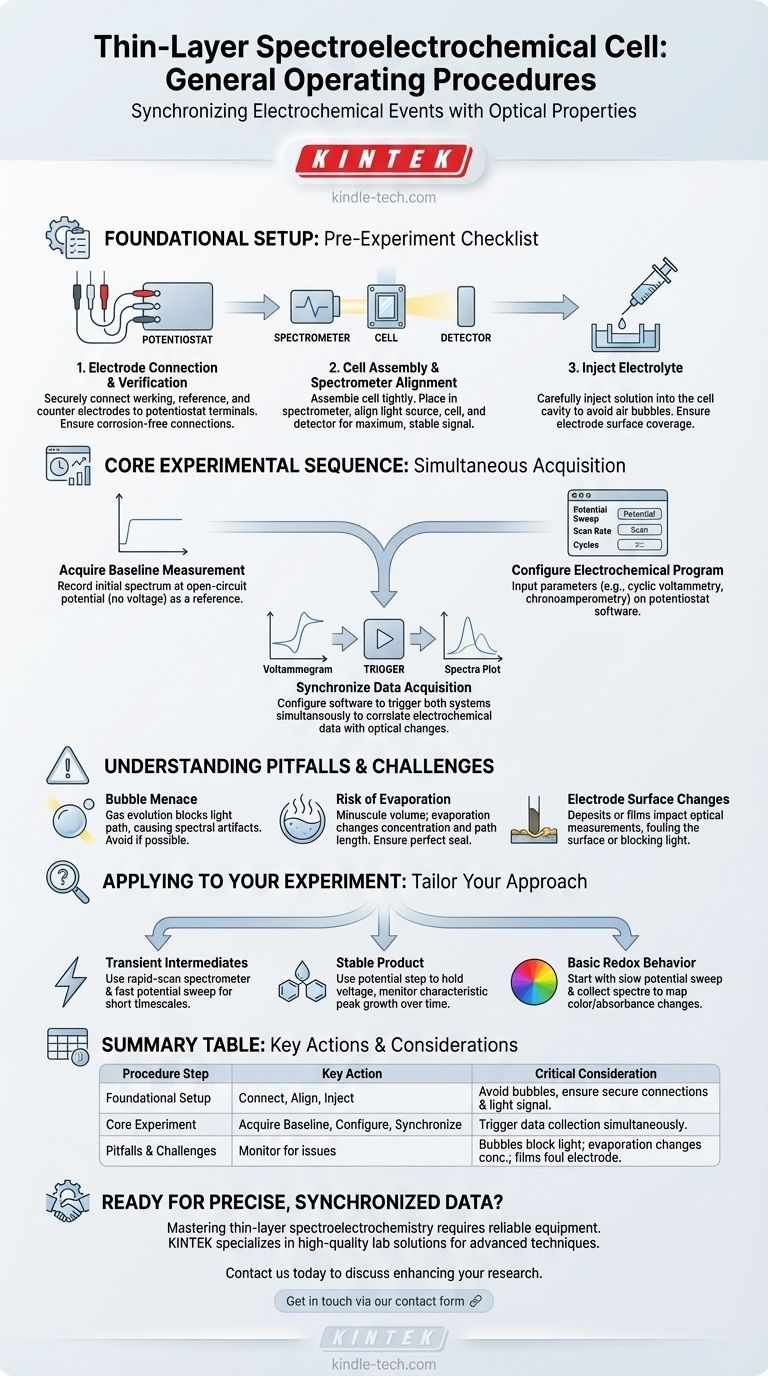
Foundational Setup: The Pre-Experiment Checklist
Before applying any potential, a rigorous setup procedure is essential for acquiring clean, reproducible data. This phase ensures that both the electrochemical and spectroscopic systems are functioning correctly and are properly aligned.
H3: Electrode Connection and Verification
First, connect the working, reference, and counter electrode leads from the cell to their corresponding terminals on the electrochemical workstation (potentiostat). Incorrect wiring is a common error that will invalidate your results. Ensure all connections are secure and free of corrosion.
H3: Cell Assembly and Spectrometer Alignment
Assemble the thin-layer cell according to the manufacturer's instructions, ensuring it is tightly sealed. Place the assembled cell into the spectrometer's sample holder. You must then align the light source, the cell, and the detector to achieve a maximum, stable light signal through the cell's transparent window.
H3: Injecting the Electrolyte
Using a syringe, carefully inject the electrolyte solution into the cell's small-volume cavity. The key is to do this slowly and methodically to avoid introducing air bubbles, which will scatter light and ruin your spectroscopic measurements. Ensure the solution completely covers the working electrode surface.
The Core Experimental Sequence
Once the cell is physically prepared and aligned, you can proceed with the combined measurement. The goal is to capture two data streams—one electrochemical, one spectroscopic—at the exact same time.
H3: Acquiring a Baseline Measurement
Before starting the electrochemical scan, you must record a baseline. This involves taking an initial spectrum of the solution at the open-circuit potential (when no voltage is applied). This initial spectrum serves as the reference against which all subsequent spectral changes will be measured.
H3: Configuring the Electrochemical Program
On the potentiostat software, input the parameters for your experiment. This could be a potential sweep (cyclic voltammetry), a potential step (chronoamperometry), or a constant current application. Define the starting potential, final potential, scan rate, and number of cycles as required by your experimental design.
H3: Synchronizing Data Acquisition
This is the most critical step. Configure your software to trigger both the potentiostat and the spectrometer to begin recording simultaneously. As the potential is swept or stepped, the spectrometer will continuously acquire spectra, allowing you to create a direct correlation between the electrochemical data (the voltammogram) and the optical changes (the spectra).
Understanding the Pitfalls and Challenges
Thin-layer spectroelectrochemistry is a powerful technique, but it is sensitive to several common sources of error. Awareness of these issues is key to troubleshooting and obtaining high-quality data.
H3: The Bubble Menace
Gas evolution (bubble formation) on the electrode surface is a frequent byproduct of electrochemical reactions. In a thin-layer cell, these bubbles can block the light path, causing major artifacts in your spectra. If possible, choose a potential window where gas evolution does not occur.
H3: The Risk of Evaporation
The electrolyte volume in a thin-layer cell is minuscule. Even minor evaporation during a long experiment can change the concentration of your analyte and the optical path length, leading to inaccurate results. Ensure your cell is perfectly sealed before you begin.
H3: Electrode Surface Changes
As mentioned in basic electrochemical procedures, reactions can form deposits or films on the electrode surface. In spectroelectrochemistry, you must consider how this film impacts the optical measurement. A new deposit may be the species you want to study, or it could be an unwanted side product that fouls the surface and blocks the light path.
Applying This to Your Experiment
Your specific procedure will depend on your research question. Use the following guidelines to tailor your approach.
- If your primary focus is identifying transient intermediates: Use a rapid-scan spectrometer and a fast potential sweep to capture spectral changes that occur on a short timescale.
- If your primary focus is quantifying a stable product: Use a potential step to hold the system at a voltage where the product is formed, and monitor the growth of its characteristic spectral peaks over time.
- If your primary focus is establishing basic redox behavior: Start with a slow potential sweep while collecting spectra to create a clear, high-resolution map of how the substance's color or absorbance changes with its oxidation state.
Your goal is to transform two separate datasets into a single, unified story about your material's behavior.
Summary Table:
| Procedure Step | Key Action | Critical Consideration |
|---|---|---|
| Foundational Setup | Connect electrodes, align cell, inject electrolyte. | Avoid air bubbles; ensure secure connections and maximum light signal. |
| Core Experiment | Acquire baseline, configure potentiostat, synchronize acquisition. | Trigger electrochemical and spectroscopic data collection simultaneously. |
| Pitfalls & Challenges | Monitor for bubbles, evaporation, and surface changes. | Bubbles block light; evaporation alters concentration; deposits foul the electrode. |
Ready to achieve precise, synchronized data in your lab?
Mastering thin-layer spectroelectrochemistry requires reliable equipment and expert support. At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to advanced electrochemical and spectroscopic techniques. Our team can help you select the right components and optimize your setup for clean, reproducible results.
Contact us today to discuss how our solutions can enhance your research and streamline your experimental workflow. Get in touch via our contact form to speak with an expert.
Visual Guide
Related Products
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- H Type Electrolytic Cell Triple Electrochemical Cell
People Also Ask
- What are the opening configurations for the non-sealed and sealed versions of the electrolysis cell? Optimize Your Electrochemical Setup
- What is the typical volume range for a single chamber in an H-type electrolytic cell? A Guide from 8 mL to 1000+ mL
- Why must PEC reactor windows have high mechanical strength? Ensuring Safety and Integrity in Solar Energy Conversion
- What are the advantages of using quartz glass as the material for an electrocatalytic oxidation reactor cell?
- What is the difference between an electrolytic cell and an electrochemical cell? Understand the Two Sides of Energy Conversion
- What is the H type photoelectrochemical cell? A Guide to Isolating & Studying Light-Driven Reactions
- Why are zirconia-polysulfone composite materials frequently utilized as diaphragms in alkaline water electrolysis?
- Why is a three-electrode electrochemical cell system necessary for Ni-Cr alloy corrosion kinetics? Expert Analysis


















