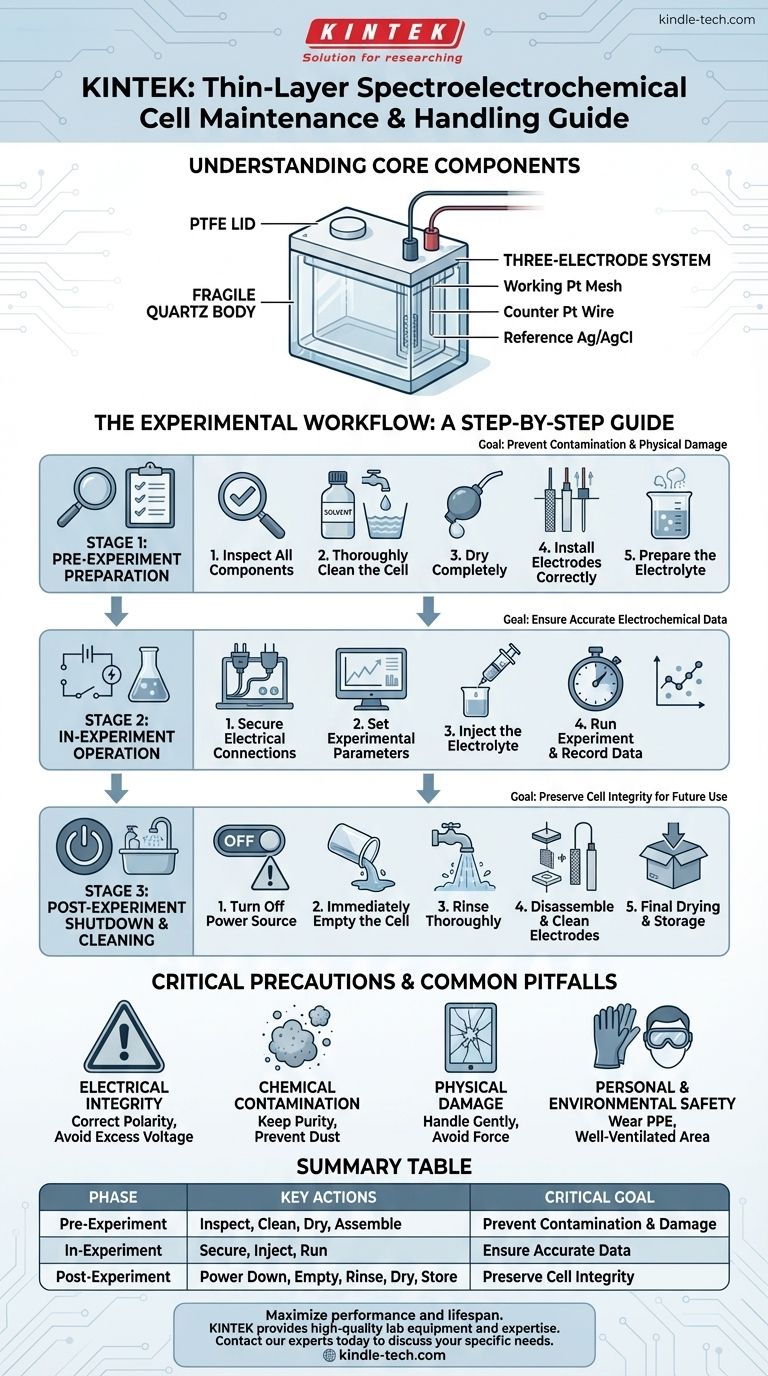
Proper handling of a thin-layer spectroelectrochemical cell is a matter of precision, not preference. The core procedures revolve around three distinct phases: meticulous pre-experiment preparation involving inspection and cleaning; careful in-experiment operation with correct electrical connections; and immediate post-experiment shutdown, which includes thorough rinsing and proper drying before storage. Adhering to this lifecycle is critical for both the accuracy of your results and the longevity of this delicate instrument.
A thin-layer spectroelectrochemical cell's power lies in its sensitive design, featuring a fragile quartz body and precise electrodes. This design demands a disciplined approach to handling and maintenance to prevent physical damage, avoid chemical contamination, and ultimately ensure the integrity of your experimental data.

Understanding the Core Components
Before diving into procedures, it's essential to understand the materials you are working with. This context informs every handling decision you make. The cell's design is a balance of optical transparency and chemical inertness.
The Quartz Cell Body
The main body is typically crafted from integrally polished quartz, chosen for its transparency across a wide spectral range. It is often assembled without adhesives, making it a single, fragile unit. Always handle it with extreme care, as any cracks or chips will render it unusable.
The PTFE Lid
The lid is made of polytetrafluoroethylene (PTFE), a highly inert polymer. This material ensures that the lid will not react with your electrolyte, whether you are using aqueous or non-aqueous systems.
The Three-Electrode System
A standard configuration includes a platinum mesh working electrode, a platinum wire counter electrode, and a silver/silver chloride (Ag/AgCl) reference electrode. The integrity and cleanliness of these electrodes are paramount for achieving accurate and reproducible electrochemical measurements.
The Experimental Workflow: A Step-by-Step Guide
Following a consistent workflow is the most effective way to protect the cell and ensure high-quality data. This process can be broken down into three clear stages.
Phase 1: Pre-Experiment Preparation
- Inspect All Components: Before assembly, carefully examine the quartz body for any cracks or damage. Check the electrodes for signs of wear, pitting, or contamination.
- Thoroughly Clean the Cell: Use a suitable solvent to remove any residues from previous experiments or storage. Rinse it multiple times with distilled or deionized water to eliminate all impurities.
- Dry Completely: Ensure the cell body is completely dry before assembly. Any residual moisture can dilute your electrolyte and skew results.
- Install Electrodes Correctly: Carefully insert the working, counter, and reference electrodes into their designated positions. Ensure they are seated properly to establish good electrical contact without stressing the quartz body.
- Prepare the Electrolyte: Prepare your electrolyte solution as required by your experimental protocol. If necessary, perform pre-treatments such as deoxygenation by bubbling an inert gas through the solution before injection.
Phase 2: In-Experiment Operation
- Secure Electrical Connections: Connect the electrode leads securely to your electrochemical workstation. Double-check that the working, counter, and reference electrodes are connected to the correct terminals.
- Set Experimental Parameters: Configure the appropriate electrochemical parameters, such as potential scan range and current limits, on your workstation.
- Inject the Electrolyte: Carefully inject the prepared electrolyte into the cell.
- Run Experiment and Record Data: Activate the equipment to begin the experiment, monitoring the process and recording all relevant data.
Phase 3: Post-Experiment Shutdown and Cleaning
- Turn Off the Power Source: This is a critical first step. Always turn off the workstation before disconnecting any leads to prevent electrical arcing, which can damage both the instrument and the cell's electrodes.
- Immediately Empty the Cell: Remove the electrolyte from the cell as soon as the experiment is complete.
- Rinse Thoroughly: Immediately rinse the cell interior multiple times with distilled or deionized water to remove residual electrolyte and reaction byproducts before they can dry and adhere to the surfaces.
- Disassemble and Clean Electrodes: Carefully remove the electrodes. Clean them according to their specific requirements to prepare them for the next use or for storage.
- Final Drying and Storage: Ensure the cell body, electrodes, and all components are completely dry. Store them in a clean, dry environment, protected from dust and moisture. For long-term storage, the cell should be empty and sealed if possible.
Critical Precautions and Common Pitfalls
Avoiding common mistakes is just as important as following the correct procedures. Be mindful of these key risks.
Electrical Integrity
Ensure the correct polarity for all electrode connections. Reversing the anode and cathode can lead to incorrect data and potential damage. Furthermore, avoid applying excessively high voltage, as this can cause uncontrolled electrolyte decomposition or permanently damage the electrodes.
Chemical Contamination
Prevent environmental contaminants like dust and other airborne particles from entering the cell, as they can interfere with your electrochemical reactions. Purity is paramount.
Physical Damage
The quartz body is the most vulnerable component. Never apply force when assembling or disassembling the cell. Always handle it gently and store it where it cannot be knocked over or struck by other objects.
Personal and Environmental Safety
When working with corrosive or toxic electrolytes, always wear appropriate personal protective equipment (PPE), including gloves and safety glasses. Ensure you are working in a well-ventilated area and take all necessary precautions to prevent spills and leaks.
Making the Right Choice for Your Goal
Your specific priorities will dictate which aspects of this process require the most attention.
- If your primary focus is maximizing data accuracy: Meticulous cleaning between experiments and proper electrolyte preparation, including deoxygenation, are your most critical steps.
- If your primary focus is ensuring equipment longevity: Gentle physical handling of the quartz body and avoiding excessive voltage during operation are non-negotiable.
- If your primary focus is maintaining a safe lab environment: Always power down before disconnecting electrodes and consistently use the correct PPE for your specific electrolyte.
Ultimately, a disciplined and repeatable process is the foundation of reliable spectroelectrochemical analysis.
Summary Table:
| Phase | Key Actions | Critical Goal |
|---|---|---|
| Pre-Experiment | Inspect, clean, dry, assemble correctly | Prevent contamination & physical damage |
| In-Experiment | Secure connections, inject electrolyte, run | Ensure accurate electrochemical data |
| Post-Experiment | Power down, empty, rinse, dry, store | Preserve cell integrity for future use |
Maximize the performance and lifespan of your laboratory equipment. Proper maintenance is key to reliable results. KINTEK specializes in high-quality lab equipment and consumables, providing the tools and expertise to support your precise spectroelectrochemical work. Contact our experts today to discuss your specific lab needs and ensure your experiments run smoothly.
Visual Guide
Related Products
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Super Sealed Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What types and sizes of electrodes are typically configured with a thin-layer spectroelectrochemical cell? Standard Setup for Accurate Analysis
- What general precautions should be taken when using a thin-layer spectroelectrochemical cell? Ensure Accurate Results and Equipment Safety
- Which electrode types are compatible with thin-layer spectroelectrochemical cells? Optimize Your Hardware Fit
- What are the physical dimensions of the thin-layer spectroelectrochemical cell body and its slit? Key Specs for Your Lab
- What are the dimensions for thin-layer spectroelectrochemical cells? Optimize Your Lab's Optical Path Length



















