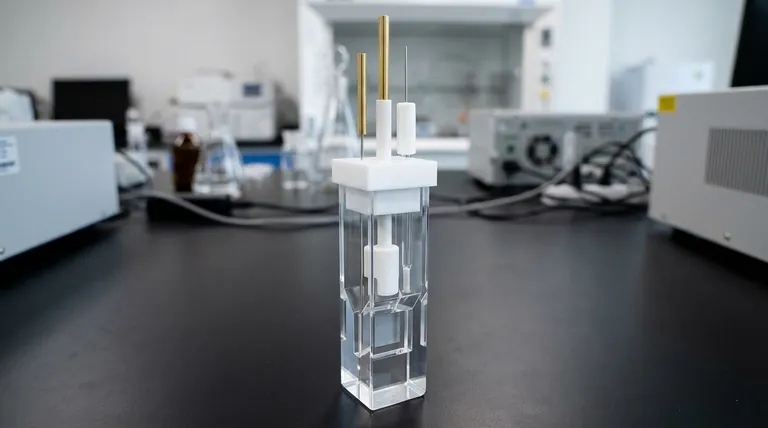
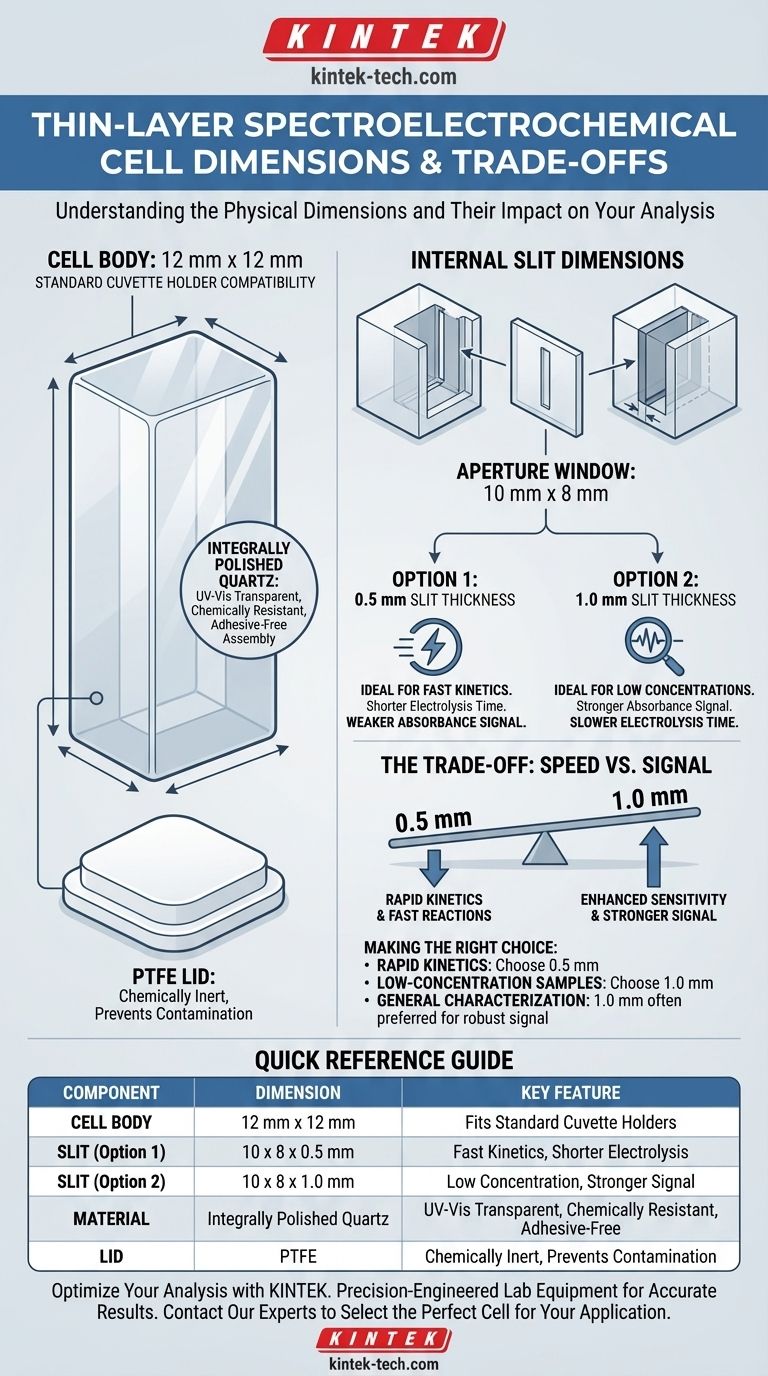
The standard thin-layer spectroelectrochemical cell body has a square footprint of 12 mm by 12 mm. This body is designed to accommodate one of two available slit dimensions, which define the internal volume and optical path length: 10 x 8 x 0.5 mm or 10 x 8 x 1.0 mm.
The cell's 12x12 mm external dimension is intentionally designed for compatibility with standard spectrophotometer cuvette holders. The critical choice between the 0.5 mm and 1.0 mm slit thickness is a trade-off between the speed of the electrochemical experiment and the strength of the resulting spectroscopic signal.
Deconstructing the Cell's Dimensions
To effectively use this cell, it is crucial to understand what each dimension represents in the context of a spectroelectrochemical experiment.
The Cell Body (12 x 12 mm)
The 12 mm by 12 mm outer dimension is the standard size for a conventional spectroscopic cuvette. This ensures the cell body fits directly into the cuvette holder of most commercial UV-Vis spectrophotometers without needing special adapters.
This body is typically machined from a single piece of integrally polished quartz. This material is transparent across the UV and visible spectrum, making it ideal for spectroscopic analysis.
The Internal Slit (10 x 8 x 0.5/1.0 mm)
The slit defines the internal chamber where the sample is held and the experiment occurs. These dimensions correspond to the height, width, and, most importantly, the thickness of this chamber.
While the 10 mm and 8 mm dimensions define the aperture window, the third dimension (0.5 mm or 1.0 mm) is the most critical parameter. It represents the optical path length for spectroscopy and the solution thickness for electrochemistry.
Understanding the Trade-offs: 0.5 mm vs. 1.0 mm Path Length
The choice between the 0.5 mm and 1.0 mm thick slit is not arbitrary. It directly impacts both the electrochemical and spectroscopic aspects of your measurement, presenting a clear trade-off.
The Case for the 0.5 mm Slit
A thinner, 0.5 mm layer is ideal for experiments where speed is critical.
Because the volume is small and the distance between electrodes is minimal, the complete electrolysis of the species in the solution occurs very rapidly. This is advantageous for studying fast reaction kinetics.
The primary drawback is a weaker signal. According to the Beer-Lambert law (A = εbc), a shorter path length (b) results in a lower absorbance reading for a given concentration, which can be a limitation for dilute samples.
The Case for the 1.0 mm Slit
A thicker, 1.0 mm layer is chosen when the spectroscopic signal is the priority.
The longer path length effectively doubles the absorbance signal compared to the 0.5 mm cell for the same sample concentration. This makes it superior for analyzing very dilute solutions or species with a low molar absorptivity.
The trade-off is time. The electrolysis of the entire solution volume will take significantly longer, as ions must diffuse across a greater distance to the working electrode.
Material and Construction Implications
The physical dimensions are only part of the story. The materials used are chosen to ensure data integrity.
Integrally Polished Quartz
Using quartz that is transparent on all four sides provides maximum optical access and durability. Its high resistance to chemical attack and thermal shock is essential for electrochemical studies.
Adhesive-Free Assembly
The cell is assembled without adhesives. This is a critical feature that prevents chemical contaminants from leaching out of glues or epoxies, which could interfere with sensitive electrochemical measurements or contaminate the sample.
PTFE Lid
The lid is made from Polytetrafluoroethylene (PTFE), a material known for its extreme chemical inertness. This ensures the lid will not react with the solvents, electrolytes, or analytes used in your experiment.
Making the Right Choice for Your Experiment
Selecting the correct slit dimension is a function of your primary experimental goal.
- If your primary focus is on rapid kinetics or fast reactions: Choose the 0.5 mm slit to minimize the time required for complete electrolysis.
- If your primary focus is analyzing low-concentration samples: Choose the 1.0 mm slit to maximize the optical path length and achieve a stronger absorbance signal.
- If you are performing general characterization with moderate concentrations: Either path length may be suitable, but the 1.0 mm cell often provides a more robust signal for general-purpose use.
Understanding these dimensions empowers you to select the precise cell configuration that aligns perfectly with your analytical goals.
Summary Table:
| Component | Dimension | Key Feature |
|---|---|---|
| Cell Body | 12 mm x 12 mm | Fits standard spectrophotometer cuvette holders |
| Internal Slit (Option 1) | 10 mm x 8 mm x 0.5 mm | Ideal for fast kinetics, shorter electrolysis time |
| Internal Slit (Option 2) | 10 mm x 8 mm x 1.0 mm | Ideal for low-concentration samples, stronger signal |
| Material | Integrally polished quartz | UV-Vis transparent, chemically resistant, adhesive-free assembly |
| Lid | PTFE | Chemically inert, prevents contamination |
Optimize your spectroelectrochemical analysis with the right equipment from KINTEK.
Choosing the correct cell dimensions is crucial for achieving accurate and reliable data. Whether your priority is rapid kinetics with the 0.5 mm slit or enhanced sensitivity with the 1.0 mm slit, KINTEK provides high-quality, precision-engineered lab equipment to meet your specific research needs.
Our thin-layer spectroelectrochemical cells are crafted from integrally polished quartz and feature adhesive-free assembly for uncontaminated results, helping you advance your research with confidence.
Ready to select the perfect cell for your application? Contact our experts today to discuss your requirements and discover how KINTEK's solutions can enhance your laboratory's capabilities.
Visual Guide
Related Products
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What are the dimensions for thin-layer spectroelectrochemical cells? Optimize Your Lab's Optical Path Length
- For what types of systems, temperature ranges, and sealing configurations is the thin-layer spectroelectrochemical cell designed? Ideal for Aqueous and Non-Aqueous Analysis
- What are the necessary preparation steps before using a thin-layer spectroelectrochemical cell? A Guide to Reliable Results
- What preparation steps are required before initiating an experiment with the thin-layer spectroelectrochemical cell?
- What types and sizes of electrodes are typically configured with a thin-layer spectroelectrochemical cell? Standard Setup for Accurate Analysis



















