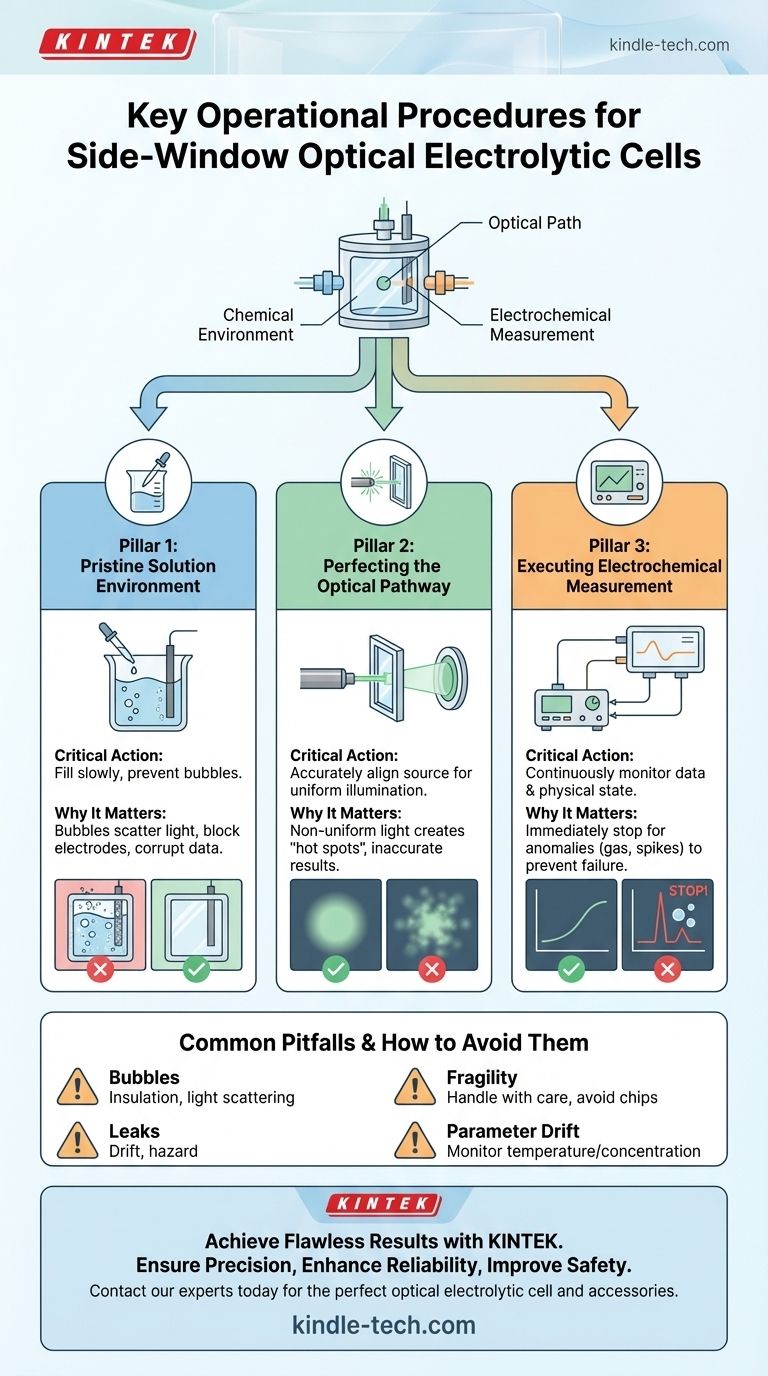
To correctly operate a side-window optical electrolytic cell during an experiment, you must first carefully fill the cell with electrolyte, ensuring no bubbles form on the window or electrode. Next, precisely align your light source with the side window to achieve uniform illumination across the working electrode's surface. Finally, initiate your measurement while continuously monitoring both the instrument data and the physical state of the cell, ready to stop immediately if any anomalies appear.
Success with an optical electrolytic cell is not about following a simple checklist. It requires simultaneously managing three critical systems: the chemical environment within the cell, the optical path from the source to the electrode, and the electrochemical measurement itself. A failure in any one of these areas will compromise your results.

The Three Pillars of a Successful Experiment
A reliable photoelectrochemical measurement depends on meticulous control over the cell's environment and instrumentation. Each step in the procedure is designed to eliminate a specific variable that could otherwise corrupt your data.
Pillar 1: Establishing a Pristine Solution Environment
Before any measurement can begin, the cell must contain a pure, bubble-free electrolyte.
Slowly introduce the electrolyte into the cell through its designated opening. A rapid pour will introduce air and create bubbles.
Bubbles are a critical point of failure. They can adhere to the optical window, scattering the incoming light, or block the electrode surface, creating an insulating layer that prevents a uniform reaction.
If bubbles do appear, gently tap the side of the cell to dislodge them. Ensure they are fully cleared from the window and all three electrode surfaces before proceeding.
Pillar 2: Perfecting the Optical Pathway
The unique feature of this cell is its optical window, which requires careful setup to be effective.
You must accurately align your light source, such as a laser or solar simulator, with the side window. The goal is to have the light beam pass through the center of the window.
Adjust your optical system to ensure the light spot uniformly illuminates the entire surface of the working electrode. Non-uniform illumination will create "hot spots" and result in an inaccurate and non-repeatable current response.
Pillar 3: Executing the Electrochemical Measurement
With the cell prepared and aligned, you can begin the experiment while maintaining active observation.
Correctly connect the cell's electrodes to your potentiostat or other instruments. Double-check that the working, reference, and counter electrode leads are connected to the proper terminals.
Set your experimental parameters, such as voltage, current, and time, according to your research goals.
Begin the measurement and continuously monitor both the software output and the physical cell. Watch for unexpected spikes or dips in the data curves.
Simultaneously, observe the cell for physical changes like excessive gas generation, color changes in the electrolyte, or precipitate formation. These are signs of unexpected side reactions.
If you observe any significant data anomalies or abnormal physical phenomena, stop the experiment immediately to diagnose the issue.
Common Pitfalls and How to Avoid Them
Even with a perfect procedure, several common issues can compromise your experiment. Being aware of them is the first step to preventing them.
The Bubble Problem
Bubbles are more than an annoyance; they actively invalidate data. A bubble on the electrode creates an insulating patch, artificially lowering the measured current. A bubble on the window scatters light, reducing the photon flux and leading to an underestimation of photo-efficiency.
Electrolyte Leakage
A poorly sealed cell is a major source of error. Leaks can alter the concentration of the electrolyte over time, causing drift in your measurements. Leaked electrolyte also poses a safety hazard and can damage surrounding equipment.
The Fragility Factor
Most optical cells are constructed from quartz or glass to ensure transparency. They must be handled with care to prevent chips or cracks that can lead to leaks or catastrophic failure. Even cells made from durable materials like PTFE have fragile optical windows.
Parameter Drift
Your results are only as good as your control over experimental conditions. Unmonitored changes in temperature or concentration during a long experiment can introduce significant artifacts, making it impossible to draw accurate conclusions.
How to Apply This to Your Research
Your specific experimental goal will determine which procedural details require the most attention.
- If your primary focus is quantitative analysis: Your top priority is the perfect, repeatable alignment of the optical path and a completely bubble-free electrode surface.
- If your primary focus is screening new materials: Your top priority is maintaining absolute consistency in your procedure—from filling to alignment—for every sample to ensure a fair comparison.
- If your primary focus is long-duration stability testing: Your top priority is ensuring the cell is perfectly sealed to prevent leaks and monitoring for any parameter drift, like temperature, over the course of the experiment.
- If your primary focus is safety and initial setup: Your top priority is ensuring the cell is clean, undamaged, and handled carefully, and that all personal protective equipment is used to prevent contact with the electrolyte.
By mastering control over these key variables, you can ensure your measurements are not only accurate but also truly insightful.
Summary Table:
| Key Operational Step | Critical Action | Why It Matters |
|---|---|---|
| Filling the Cell | Introduce electrolyte slowly to prevent bubbles. | Bubbles on the window or electrode scatter light and insulate surfaces, corrupting data. |
| Optical Alignment | Align light source to uniformly illuminate the working electrode. | Non-uniform illumination creates 'hot spots,' leading to inaccurate and non-repeatable results. |
| Initiating Measurement | Monitor instrument data and cell's physical state continuously. | Allows for immediate stoppage if anomalies (e.g., gas generation, data spikes) occur, preventing failed experiments. |
Achieve Flawless Photoelectrochemical Results with KINTEK
Mastering the delicate balance of chemistry, optics, and electrochemistry is essential for successful experiments with side-window optical electrolytic cells. Whether you are developing new materials, conducting quantitative analysis, or running long-term stability tests, having the right equipment is paramount.
KINTEK specializes in premium lab equipment and consumables designed to meet the rigorous demands of electrochemical research. Our products help you:
- Ensure Precision: Achieve the bubble-free environments and perfect optical alignment critical for accurate data.
- Enhance Reliability: Work with durable, well-sealed cells to prevent leaks and parameter drift during long experiments.
- Improve Safety: Handle electrolytes and fragile components with confidence using robust equipment.
Stop struggling with inconsistent results. Let KINTEK's expertise in laboratory solutions empower your research.
Contact our experts today to find the perfect optical electrolytic cell and accessories for your specific application.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell for Coating Evaluation
- H Type Electrolytic Cell Triple Electrochemical Cell
- Thin-Layer Spectral Electrolysis Electrochemical Cell
People Also Ask
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy