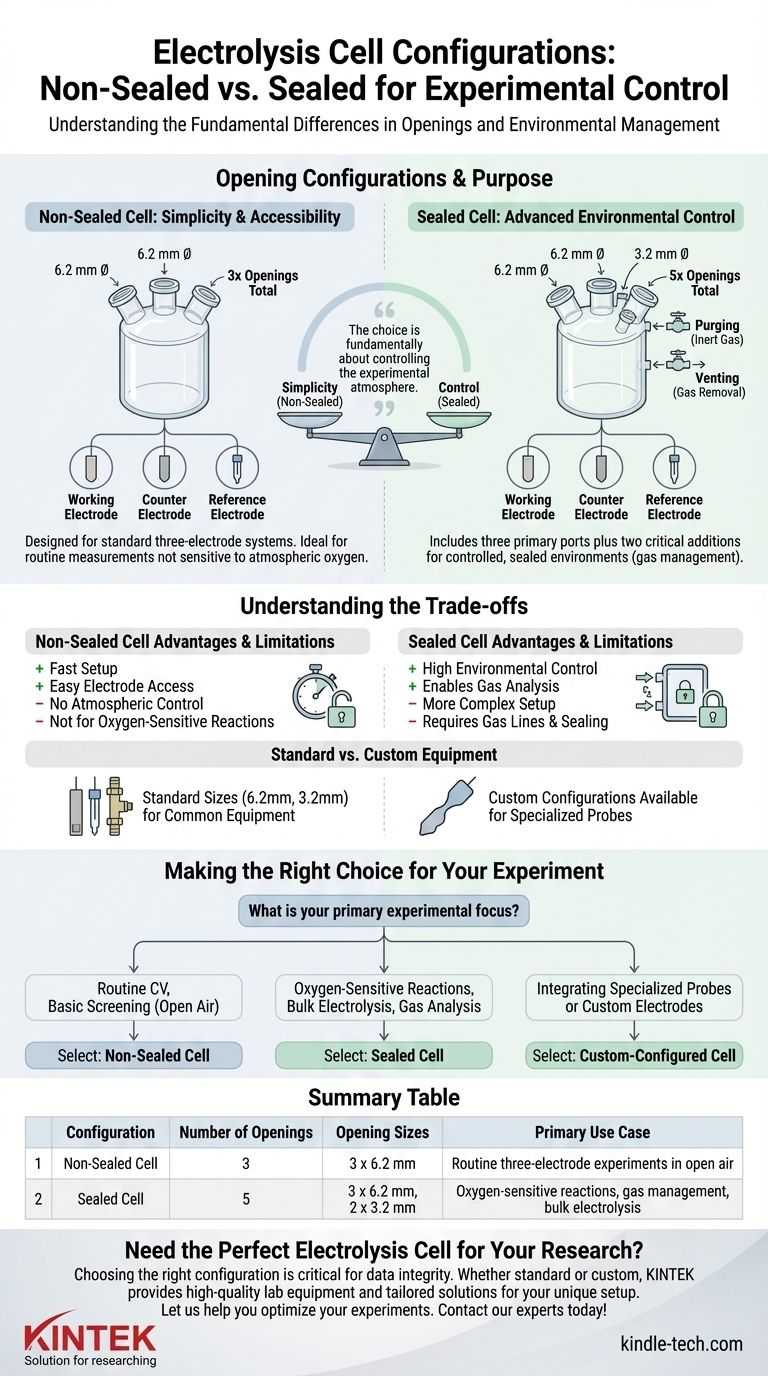
The fundamental difference in opening configurations is driven by experimental control. A standard non-sealed electrolysis cell features three openings, each with a 6.2 mm diameter. The sealed version provides more advanced control with five openings: three at 6.2 mm and two additional, smaller ports at 3.2 mm.
The choice between a non-sealed and sealed cell is fundamentally about controlling the experimental atmosphere. While the non-sealed version offers simplicity for open-air tests, the sealed version's additional ports are specifically designed for experiments that require an inert environment or precise gas management.

The Purpose of Each Configuration
The number and size of the openings are not arbitrary; they directly relate to the components of a standard electrochemical experiment and the level of environmental control required.
The Non-Sealed Cell: Simplicity and Accessibility
The three 6.2 mm openings in a non-sealed cell are designed to accommodate a standard three-electrode system.
Typically, these ports are used for the working electrode, the counter electrode, and the reference electrode.
This configuration is ideal for routine electrochemical measurements where the reaction is not sensitive to atmospheric oxygen or where precise control of the gaseous headspace is unnecessary.
The Sealed Cell: Advanced Environmental Control
The sealed cell retains the three primary 6.2 mm openings for the same three-electrode system.
The critical additions are the two smaller 3.2 mm openings. These ports are essential for creating a controlled, sealed environment.
Their primary functions are for purging and venting. One port is used to introduce an inert gas, such as argon or nitrogen, to remove oxygen from the headspace and solution. The other port serves as a vent for this purging process or for gases evolved during the reaction.
Understanding the Trade-offs
Choosing a cell is a practical decision based on experimental needs. Each design presents a clear set of advantages and limitations.
Simplicity vs. Control
The non-sealed cell offers maximum simplicity. Setup is fast, and electrodes can be easily inserted or adjusted. Its significant drawback is the complete lack of atmospheric control.
The sealed cell provides the high degree of control necessary for studying oxygen-sensitive redox processes or for experiments involving gas evolution analysis (e.g., hydrogen or oxygen evolution reactions). This control comes at the cost of a more complex setup requiring gas lines, septa, and careful sealing.
Standard vs. Custom Equipment
The standard opening sizes (6.2 mm and 3.2 mm) are designed for common commercial electrodes and fittings.
However, many experiments involve non-standard equipment, such as spectroelectrochemical probes, larger custom electrodes, or unique sampling lines. The availability of custom configurations is a critical feature, allowing you to tailor the cell top to your specific experimental hardware.
Making the Right Choice for Your Experiment
Your experimental goal should be the deciding factor when selecting a cell configuration.
- If your primary focus is routine cyclic voltammetry or basic material screening in an open atmosphere: The non-sealed cell provides the necessary ports for a three-electrode system with maximum simplicity.
- If your primary focus is studying oxygen-sensitive reactions, performing bulk electrolysis, or analyzing gaseous products: The sealed cell is essential, as its additional ports allow for deaeration and proper gas management.
- If your primary focus is integrating specialized probes or custom-built electrodes: A custom-configured cell is the most reliable option to ensure all components fit correctly without compromise.
Selecting the correct cell configuration is the foundational step toward ensuring the integrity and reproducibility of your electrochemical data.
Summary Table:
| Configuration | Number of Openings | Opening Sizes | Primary Use Case |
|---|---|---|---|
| Non-Sealed Cell | 3 | 3 x 6.2 mm | Routine three-electrode experiments in open air |
| Sealed Cell | 5 | 3 x 6.2 mm, 2 x 3.2 mm | Oxygen-sensitive reactions, gas management, and bulk electrolysis |
Need the Perfect Electrolysis Cell for Your Research?
Choosing the right cell configuration is critical for data integrity and reproducibility. Whether you require a standard non-sealed cell for routine testing or a sealed cell for advanced atmospheric control, KINTEK has the solution. We specialize in high-quality lab equipment, including custom-configured electrolysis cells to fit your unique experimental setup—from specialized probes to custom electrodes.
Let us help you optimize your electrochemical experiments. Contact our experts today to discuss your specific needs and ensure you get the precise equipment for reliable results.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- How should the five-port water bath electrolytic cell be operated during an experiment? Master Precise Control for Reliable Results
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results



















