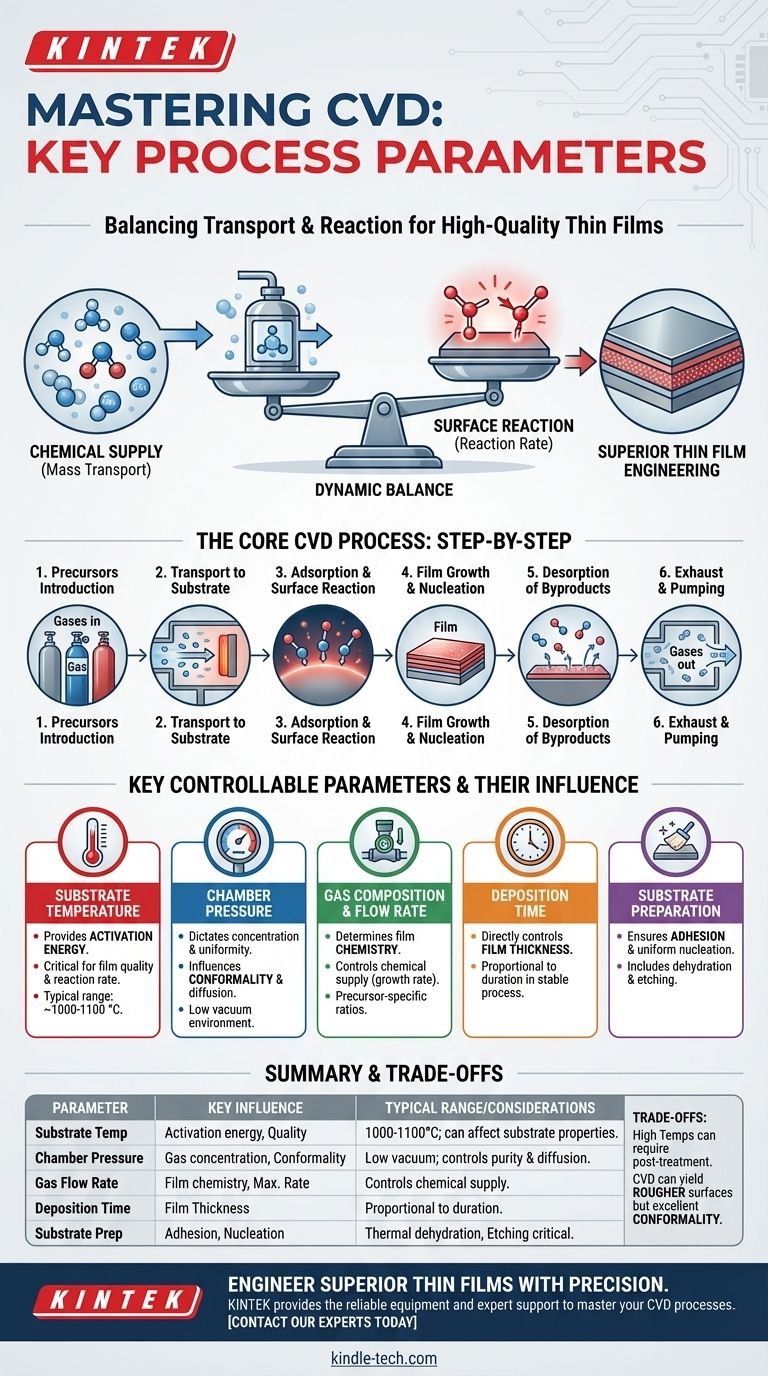
In Chemical Vapor Deposition (CVD), the primary parameters are the substrate temperature, the chamber pressure, the composition and flow rate of reactant gases (precursors), and the deposition time. These variables are meticulously controlled to govern the chemical reactions that form a solid film on a substrate, dictating the final material's thickness, quality, and properties.
The core challenge of any CVD process is not just setting these parameters, but understanding how they create a dynamic balance between two competing factors: the rate of chemical supply (mass transport) and the rate of chemical reaction at the substrate surface. Mastering this balance is the key to engineering high-quality thin films.

The Core CVD Process: A Step-by-Step Breakdown
To understand why each parameter matters, you must first visualize the fundamental journey of the atoms from a gas source to a solid film. The entire process unfolds in a sequence of physical and chemical steps.
### Introduction of Reactants (Precursors)
The process begins by introducing specific gaseous molecules, known as precursors, into a reaction chamber. These gases contain the elemental components of the desired final film material.
### Transport to the Substrate
Once inside the chamber, these precursor gases must travel from the main gas flow to the surface of the object being coated, known as the substrate. This movement is governed by pressure and gas flow dynamics.
### Adsorption and Surface Reaction
The precursor molecules physically attach (adsorb) to the heated substrate surface. The thermal energy from the substrate then provides the activation energy needed to break chemical bonds, initiating a heterogeneous surface reaction.
### Film Growth and Nucleation
The products of this surface reaction are the atoms that form the film. They diffuse across the surface to find stable growth sites, leading to the nucleation and growth of the desired solid material, layer by layer.
### Desorption of Byproducts
The chemical reactions also create unwanted gaseous byproducts. These molecules must detach (desorb) from the substrate surface and be transported away by the gas flow to prevent them from contaminating the growing film.
The Key Parameters You Control
Each step in the process is directly influenced by a set of controllable parameters. Adjusting one invariably affects the others, requiring a holistic approach to process control.
### Substrate Temperature
This is arguably the most critical parameter. Temperature provides the activation energy for the surface reactions. Higher temperatures generally increase the reaction rate, but excessively high temperatures can lead to unwanted gas-phase reactions or poor film structure. Typical ranges can be very high, often 1000-1100 °C.
### Chamber Pressure & Vacuum Level
Pressure dictates the concentration and mean free path of the gas molecules. The process is typically run in a low vacuum gaseous environment, which helps control purity by removing contaminants and influences how uniformly the precursors reach the substrate.
### Reactant Gas Composition & Flow Rate
The specific precursor gases used determine the film's chemistry. The rate at which they are pumped into the chamber controls the "supply" side of the equation, directly influencing the maximum possible growth rate.
### Deposition Time
This is the most straightforward parameter for controlling film thickness. For a stable process, the thickness of the deposited film is directly proportional to the duration of the deposition.
### Substrate Material & Preparation
The substrate is not a passive observer. Its surface chemistry must be properly prepared through steps like thermal dehydration to remove moisture or etching to remove passivation layers. This ensures the film adheres properly and grows uniformly.
Understanding the Trade-offs and Practical Implications
Controlling the CVD process involves balancing competing goals and accepting certain inherent characteristics of the technology.
### The Impact of High Temperatures
CVD often operates at temperatures that can alter the underlying substrate material. For instance, when coating hardened steel tools, the process temperature may exceed the steel's tempering point, requiring a secondary vacuum heat treatment after coating to restore hardness.
### Surface Finish Considerations
The nature of crystal growth in CVD can result in a coating that has a slightly rougher surface finish than the original substrate. This may require post-processing steps like polishing if a perfectly smooth surface is required.
### Conformal Coating Advantages
A key strength of CVD is its ability to produce conformal coatings. Because the reactants are in a gaseous state, they can penetrate and coat complex geometries, including deep holes and internal channels, with excellent uniformity—a significant advantage over line-of-sight methods like PVD.
Making the Right Choice for Your Goal
The optimal parameters depend entirely on what you are trying to achieve with your thin film.
- If your primary focus is maximizing film quality and purity: Prioritize precise substrate temperature control and thorough substrate preparation to ensure ideal surface reactions.
- If your primary focus is increasing deposition rate: Carefully increase reactant flow rates and temperature, but constantly monitor for signs of quality degradation or gas-phase reactions.
- If your primary focus is ensuring uniform coverage (conformality): Focus on managing chamber pressure and flow dynamics to ensure precursor gases can diffuse evenly across all surfaces of complex parts.
Ultimately, mastering the parameters of CVD transforms the process from a simple coating technique into a precise method for materials engineering.
Summary Table:
| Parameter | Key Influence | Typical Range/Considerations |
|---|---|---|
| Substrate Temperature | Activation energy for surface reactions; critical for film quality. | Often 1000-1100°C; can affect substrate properties. |
| Chamber Pressure | Gas molecule concentration & uniformity; influences conformality. | Low vacuum environment to control purity and diffusion. |
| Gas Composition & Flow Rate | Film chemistry and maximum possible deposition rate. | Precursor-specific; flow rate controls chemical supply. |
| Deposition Time | Directly controls final film thickness. | Proportional to thickness in a stable process. |
| Substrate Preparation | Ensures proper film adhesion and uniform nucleation. | Steps like thermal dehydration or etching are critical. |
Engineer superior thin films for your laboratory with precision.
Understanding the delicate balance of CVD parameters is the first step. Implementing them effectively requires reliable equipment and expert support. KINTEK specializes in lab equipment and consumables, providing the tools and knowledge to help you master your CVD processes—whether your goal is impeccable film quality, high deposition rates, or perfect conformal coatings on complex parts.
Contact our experts today to discuss how our solutions can meet your specific laboratory needs and elevate your materials research.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- Why does a PECVD vacuum system require both a rotary vane and turbo pump? Ensure High-Purity Coatings
- How are thin films deposited? A Guide to PVD vs. CVD Methods for Your Application
- Why is a Matching Network Indispensable in RF-PECVD for Siloxane Films? Ensure Stable Plasma and Uniform Deposition
- What is the difference between PECVD and APCVD? Choose the Right CVD Method for Your Application
- What is the difference between plasma CVD and thermal CVD? Choose the Right Method for Your Substrate



















