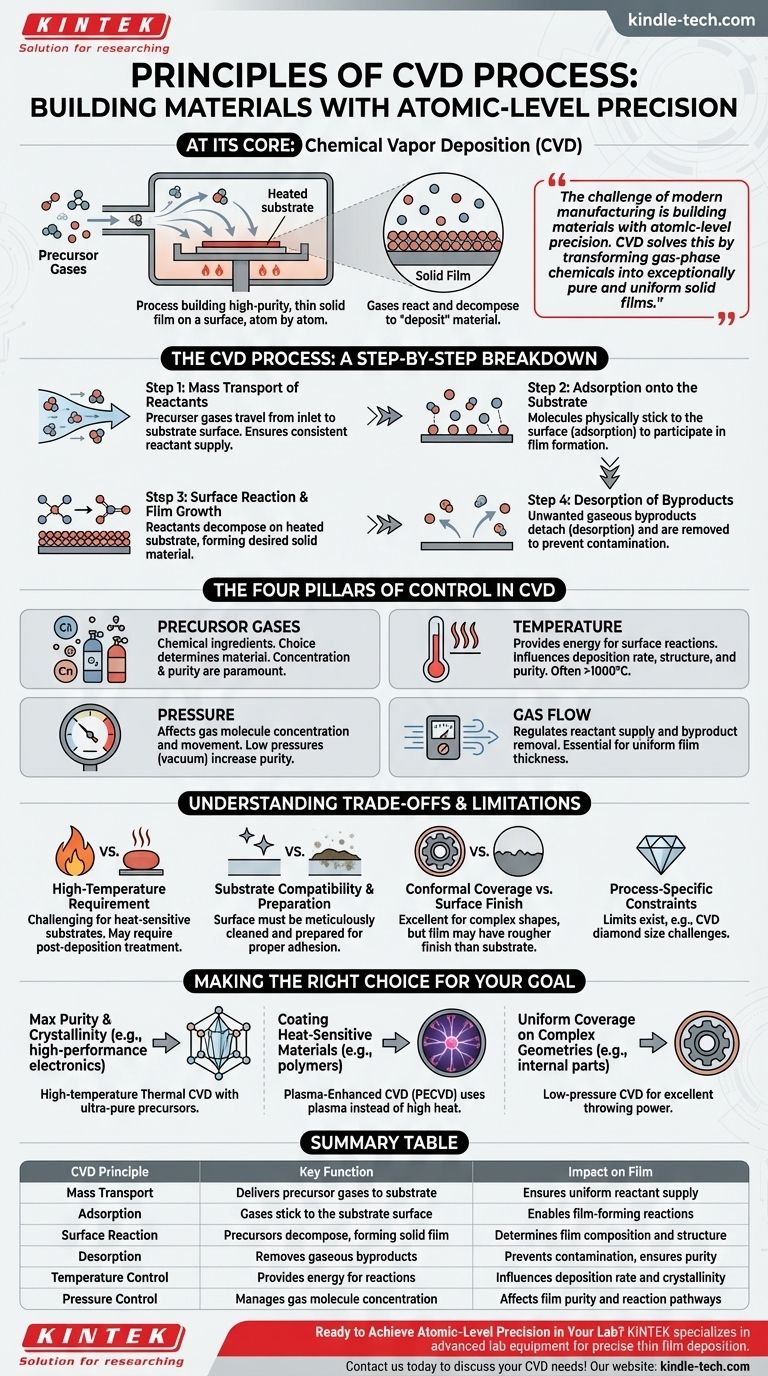
At its core, Chemical Vapor Deposition (CVD) is a process that builds a high-purity, thin solid film on a surface, atom by atom. It works by introducing specific precursor gases into a reaction chamber containing a substrate. By precisely controlling conditions like temperature and pressure, these gases react and decompose, causing the desired material to "deposit" onto the substrate, forming a new, solid layer.
The challenge of modern manufacturing is building materials with atomic-level precision. CVD solves this by transforming gas-phase chemicals into exceptionally pure and uniform solid films. The key is understanding that every parameter—from temperature to gas flow—is a lever that directly controls the final properties of the material.

The CVD Process: A Step-by-Step Breakdown
CVD is not a single event but a sequence of physical and chemical steps. For a successful deposition, each stage must be carefully managed.
Step 1: Mass Transport of Reactants
First, the precursor gases are introduced into the reaction chamber. They must travel from the gas inlet to the surface of the substrate. This flow is managed to ensure a consistent and uniform supply of reactants across the entire substrate surface.
Step 2: Adsorption onto the Substrate
Once the gas molecules reach the substrate, they must physically stick to the surface. This process is known as adsorption. This step is critical, as only adsorbed molecules can participate in the film-forming reaction.
Step 3: Surface Reaction and Film Growth
With the reactants adsorbed on the heated substrate, chemical reactions occur. These reactions break down the precursor molecules, leaving behind the desired solid material, which bonds to the substrate and begins to form a film. This is the heart of the deposition process.
Step 4: Desorption of Byproducts
The chemical reactions that form the film also create unwanted gaseous byproducts. These byproducts must detach from the surface (desorption) and be transported away by the gas flow so they do not contaminate the growing film.
The Four Pillars of Control in CVD
The quality, thickness, and properties of the final film are not accidental. They are the direct result of controlling four fundamental parameters.
Precursor Gases
These are the chemical ingredients for the film. The choice of precursors determines the material being deposited (e.g., graphene, diamond, silicon nitride). Their concentration and purity are paramount for a high-quality result.
Temperature
Temperature provides the energy needed to drive the chemical reactions on the substrate surface. It is often the most critical parameter, influencing the deposition rate, film structure (crystalline or amorphous), and purity. Temperatures can often exceed 1000°C.
Pressure
The pressure inside the reaction chamber affects the concentration of gas molecules and how they move. Lower pressures (vacuum conditions) are often used to increase the purity of the film by removing unwanted atmospheric gases and controlling the reaction pathways.
Gas Flow
The rate and pattern of gas flow ensure that fresh precursors are continuously supplied to the substrate and that waste byproducts are efficiently removed. Proper flow design is essential for achieving a film with uniform thickness across a large area.
Understanding the Trade-offs and Limitations
While powerful, CVD is not without its challenges. Understanding its limitations is key to using it effectively.
The High-Temperature Requirement
Many CVD processes operate at extremely high temperatures. This can be a problem for substrates that cannot withstand the heat. For example, coating a hardened steel tool may require it to be heat-treated again after deposition to restore its hardness.
Substrate Compatibility and Preparation
The substrate is not a passive observer. Its surface must be meticulously cleaned and prepared to ensure the film adheres properly and grows uniformly. Any impurities, like residual oxygen or moisture, must be removed before deposition.
Conformal Coverage vs. Surface Finish
A major advantage of CVD is its ability to produce highly conformal coatings, meaning it can uniformly coat complex shapes, deep holes, and internal walls. However, the resulting film can sometimes have a slightly rougher surface finish than the original substrate.
Process-Specific Constraints
Certain CVD applications have inherent limits. For instance, while CVD can produce exceptionally pure synthetic diamonds, the process currently faces challenges in growing single crystals larger than a few carats.
Making the Right Choice for Your Goal
The optimal CVD approach depends entirely on the desired outcome. The process parameters are tuned to meet the specific requirements of the application.
- If your primary focus is maximum purity and crystallinity (e.g., high-performance electronics): You will likely use a high-temperature thermal CVD process with ultra-pure precursors and rigorous substrate cleaning.
- If your primary focus is coating heat-sensitive materials (e.g., polymers or certain metals): Plasma-Enhanced CVD (PECVD), which uses plasma instead of high heat to drive reactions, is the necessary choice.
- If your primary focus is uniform coverage on complex geometries (e.g., coating internal parts): The excellent throwing power of a low-pressure CVD process is a key advantage that other methods cannot easily match.
Ultimately, mastering CVD is about mastering the interplay between its controlling parameters to build the precise material you need.
Summary Table:
| CVD Principle | Key Function | Impact on Film |
|---|---|---|
| Mass Transport | Delivers precursor gases to substrate | Ensures uniform reactant supply |
| Adsorption | Gases stick to the substrate surface | Enables film-forming reactions |
| Surface Reaction | Precursors decompose, forming solid film | Determines film composition and structure |
| Desorption | Removes gaseous byproducts | Prevents contamination, ensures purity |
| Temperature Control | Provides energy for reactions | Influences deposition rate and crystallinity |
| Pressure Control | Manages gas molecule concentration | Affects film purity and reaction pathways |
Ready to Achieve Atomic-Level Precision in Your Lab?
KINTEK specializes in advanced lab equipment and consumables for precise thin film deposition. Whether you're working with high-temperature thermal CVD or plasma-enhanced processes for sensitive materials, our solutions ensure superior film purity, uniformity, and conformal coverage.
Contact us today to discuss how our CVD expertise can enhance your research or production outcomes!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- Laboratory CVD Boron Doped Diamond Materials
People Also Ask
- What kind of dimensional structure graphene has? Discover the Power of the 2D Material
- What are the characteristics of CVD diamond? Unlocking Superior Performance for Industrial Tools
- How thick is a sputtering target? A Guide to Specifying the Right Thickness for Your Process
- For what purposes is Chemical Vapor Deposition (CVD) considered an efficient technique? Unlock High-Performance Coatings
- What machine do I need to make diamonds? HPHT vs. CVD Equipment Explained
- What is the vapor phase deposition process? A Guide to CVD and PVD Thin-Film Coating
- What is the difference between biochar and pyrolysis? Unlocking the Process vs. Product Relationship
- How does a CVD reaction furnace contribute to NCD coatings? Precision Synthesis for Diamond-Clad High-Performance Parts



















