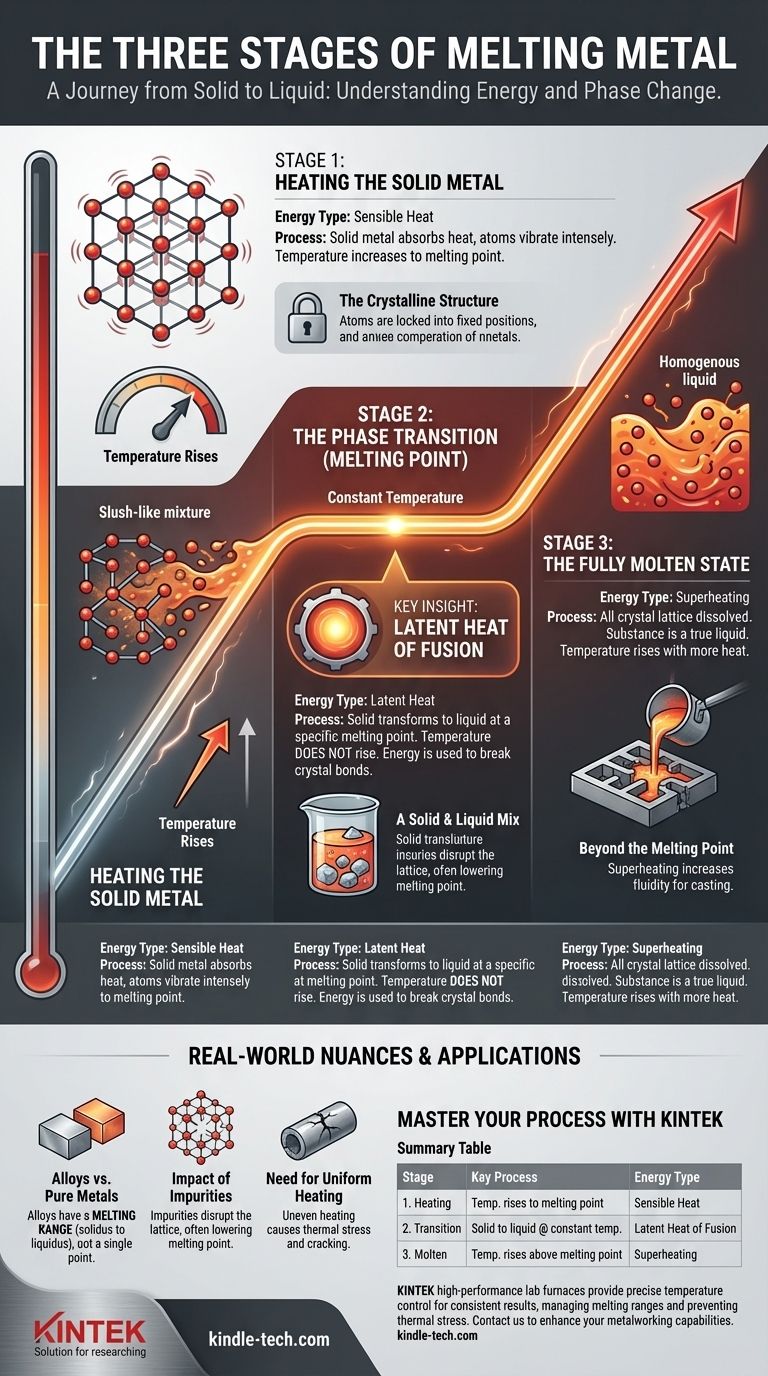
In practice, melting metal is a three-stage process. First, the solid metal absorbs heat and its temperature rises. Second, it reaches a specific melting point where it continues to absorb a great deal of energy without getting any hotter, transforming from solid to liquid. Finally, once fully molten, its temperature will rise again as more heat is applied.
The most critical insight is that melting is not just about reaching a temperature, but about supplying a specific, additional amount of energy—the latent heat of fusion—at that constant temperature to break the bonds of the metal's solid structure.

Stage 1: Heating the Solid Metal
This initial stage involves raising the temperature of the metal from its starting point up to its melting point. It is a straightforward process of energy absorption that you can easily measure with a thermometer.
The Crystalline Structure
At a microscopic level, a solid metal is a highly organized crystal lattice. Atoms are locked into fixed positions, vibrating with thermal energy. They are bonded tightly to their neighbors, giving the metal its strength and shape.
Absorbing Sensible Heat
As you apply an energy source, the atoms within this lattice vibrate more and more intensely. This increase in atomic vibration is what we measure as a rise in temperature. This form of energy is called sensible heat because we can sense it as a direct change in temperature.
Stage 2: The Phase Transition at the Melting Point
This is the most crucial and often misunderstood stage. Here, the metal undergoes its fundamental change of state from solid to liquid, a process that occurs at a constant temperature.
Reaching the Critical Temperature
Once the atomic vibrations become too violent for the crystalline bonds to hold them in place, the metal has reached its melting point. For a pure metal, this is a very specific and defined temperature (e.g., 1538°C for iron).
The Role of Latent Heat
At the melting point, something remarkable happens. Even though you continue to add heat, the temperature of the metal stops rising.
This new energy, known as the latent heat of fusion, is consumed entirely by the process of breaking the bonds of the crystal lattice. It is "latent" or hidden because it doesn't produce a temperature change.
A Solid and Liquid Mix
During this entire phase, the metal exists as a slush-like mixture of solid and liquid. The temperature will remain constant at the melting point until every last crystal has been broken down and transformed into liquid.
Stage 3: The Fully Molten State
Once the phase transition is complete, the metal behaves as a true liquid. Any further heat application will once again cause a measurable rise in temperature.
Beyond the Melting Point
With the crystal lattice completely dissolved, all the atoms are now free to move past one another. The substance is now a homogenous liquid.
Superheating the Liquid
In practical applications like casting, the molten metal is often heated to a temperature significantly above its melting point. This is called superheating. It increases the metal's fluidity and ensures it can fill a complex mold before it begins to solidify.
Understanding the Practical Complications
While the three stages provide a clean theoretical model, real-world metal melting involves important nuances.
Alloys vs. Pure Metals
Pure metals have a single, sharp melting point. Most metals we use, however, are alloys (mixtures of metals). Alloys do not have a single melting point but rather a melting range. They begin to melt at one temperature (the solidus) and become fully liquid at a higher temperature (the liquidus), existing as a slushy mix in between.
The Impact of Impurities
Impurities within a metal can disrupt its crystal structure. This almost always lowers the melting point and can create a melting range, making the metal's behavior less predictable.
The Need for Uniform Heating
Applying heat too quickly or unevenly can cause thermal stress. Parts of the metal may melt while others are still solid and expanding, which can cause cracking or warping, especially in complex parts.
How to Apply This to Your Goal
Understanding these stages allows you to control the process for your specific objective.
- If your primary focus is casting: Your goal is to get well into Stage 3, superheating the metal to ensure high fluidity for a successful pour.
- If your primary focus is welding: You are creating a localized zone that rapidly moves through all three stages to fuse components, and understanding the slushy transition state (Stage 2) is key to managing the weld pool.
- If your primary focus is academic study: The critical concept to grasp is the distinction between sensible heat (which changes temperature) and latent heat (which changes the state).
Ultimately, mastering the behavior of metal requires seeing heat not just as a measure of temperature, but as the energy that drives its fundamental transformation.
Summary Table:
| Stage | Key Process | Energy Type |
|---|---|---|
| 1. Heating | Temperature rises to melting point | Sensible Heat |
| 2. Transition | Solid to liquid at constant temperature | Latent Heat of Fusion |
| 3. Molten | Temperature rises above melting point | Superheating |
Master Your Metal Melting Process with KINTEK
Understanding the precise stages of metal melting is crucial for achieving consistent results in casting, welding, or research. KINTEK specializes in high-performance lab furnaces and equipment that deliver the precise temperature control and uniform heating needed to navigate each stage effectively—from initial heating through the critical phase transition to superheating.
Whether you're working with pure metals or complex alloys, our solutions help you avoid thermal stress, manage melting ranges, and achieve the fluidity required for perfect pours.
Ready to enhance your metalworking capabilities? Contact our experts today to find the ideal equipment for your specific application and ensure every melt is a success.
Visual Guide
Related Products
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is the role of muffle furnace in fluid mechanics? A Key Tool for Material Preparation
- What type of insulation is used in a muffle furnace? Essential Materials for High-Temperature Performance
- What apparatus is used for heating in a lab? A Guide to Choosing the Right Tool
- How do you clean a muffle furnace? A Step-by-Step Guide to Ensure Safety and Longevity
- What is the purpose of a muffle furnace in a lab? Achieve Pure, High-Temperature Heat for Your Materials



















