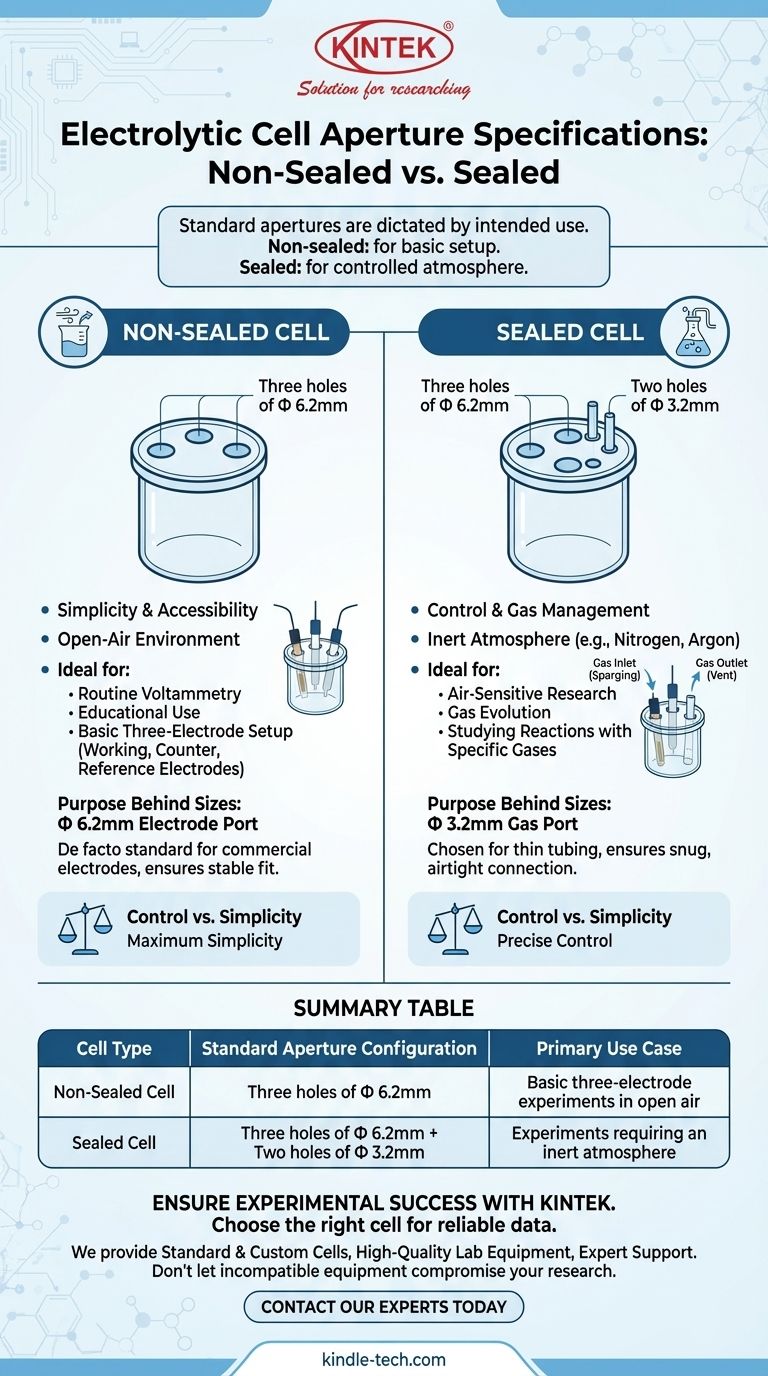
For a standard electrochemical cell, the aperture specifications are dictated by its intended use. A non-sealed cell typically has three holes of Φ 6.2mm, designed for a basic three-electrode setup. A sealed cell expands on this, featuring three holes of Φ 6.2mm and two smaller holes of Φ 3.2mm to allow for a controlled atmosphere.
The number and size of apertures on an electrolytic cell are not arbitrary; they directly reflect the experimental control required. The primary difference between sealed and non-sealed cells is the addition of dedicated ports for managing the gas environment.
Understanding the Standard Configurations
The standard aperture layout is the foundation for most electrochemical experiments. Understanding its purpose is key to selecting the right equipment.
The Non-Sealed Cell: Simplicity and Accessibility
A non-sealed cell is the workhorse for routine electrochemical analysis in an open-air environment. Its standard configuration is three holes, each with a Φ 6.2mm diameter.
These three ports are designed to accommodate a standard three-electrode system: a working electrode, a counter (or auxiliary) electrode, and a reference electrode. This setup is ideal for basic cyclic voltammetry, educational purposes, and any experiment where atmospheric exposure is not a concern.
The Sealed Cell: Control and Gas Management
A sealed cell is required for experiments that are sensitive to air or involve gaseous reactants or products. Its standard configuration includes three holes of Φ 6.2mm and two additional holes of Φ 3.2mm.
The three larger ports serve the same purpose as in the non-sealed cell—holding the electrodes. The two smaller Φ 3.2mm ports are gas ports, designed for small-diameter tubing. They allow you to purge the electrolyte with an inert gas (like nitrogen or argon) to remove dissolved oxygen or to study reactions involving specific gases.
The Purpose Behind the Sizes
Each aperture diameter is an industry-standard choice made for practical reasons, much like the ports on a computer are standardized for specific peripherals.
The Φ 6.2mm "Electrode Port"
This larger diameter is the de facto standard for most commercially available electrochemical electrodes. It provides a secure fit for the working, counter, and reference electrodes from majeur manufacturers, ensuring stability during measurement.
The Φ 3.2mm "Gas Port"
This smaller diameter is specifically chosen for the thin plastic or PTFE tubing used for gas lines. It allows for a snug, airtight connection, which is critical for maintaining the integrity of the sealed environment inside the cell. One port serves as a gas inlet (for sparging) and the other as a vent or outlet.
Common Variations and Custom Needs
While the standards cover most use cases, more complex experiments often require different layouts.
Multi-Chamber and H-Type Cells
More complex cells, such as H-type cells that separate the anode and cathode compartments, apply these same principles to each chamber. Each section may have its own set of electrode and gas ports to allow for independent control and measurement.
When to Consider Custom Apertures
You should verify dimensions and consider a custom configuration if your experiment involves non-standard components. This includes using oversized or custom-made electrodes, adding extra sensors for temperature or pH, or incorporating a Luggin capillary for precise IR drop compensation.
Understanding the Trade-offs
Choosing between a sealed and non-sealed cell is a fundamental decision that impacts your experimental capabilities and complexity.
Control vs. Simplicity
A sealed cell offers precise control over the chemical environment, which is non-negotiable for air-sensitive research. However, this comes at the cost of increased setup complexity.
A non-sealed cell offers maximum simplicity and ease of use. It is perfect for rapid screening and robust analyses but is entirely unsuitable for experiments जहां an inert atmosphere is required.
The Risk of Incompatibility
Always verify the outer diameter of your electrodes, probes, and gas tubing before purchasing a cell. While Φ 6.2mm is a common standard for electrodes, it is not universal. Assuming compatibility without checking is a frequent and costly mistake.
Making the Right Choice for Your Goal
Select your cell configuration based on the specific demands of your electrochemical analysis.
- If your primary focus is routine voltammetry or general analysis in open air: A non-sealed cell with three Φ 6.2mm ports is the most efficient and straightforward choice.
- If your primary focus is studying air-sensitive materials, gas evolution reactions, or require an inert atmosphere: A sealed cell with both Φ 6.2mm and Φ 3.2mm ports is essential for valid results.
- If your primary focus involves specialized equipment like oversized electrodes, extra sensors, or a Luggin capillary: You must confirm all component dimensions and will likely need to request a custom-configured cell.
Matching the cell's design to your experimental needs is the first step toward achieving reliable and repeatable electrochemical data.

Summary Table:
| Cell Type | Standard Aperture Configuration | Primary Use Case |
|---|---|---|
| Non-Sealed Cell | Three holes of Φ 6.2mm | Basic three-electrode experiments in open air (e.g., routine voltammetry) |
| Sealed Cell | Three holes of Φ 6.2mm + Two holes of Φ 3.2mm | Experiments requiring an inert atmosphere or gas management (e.g., air-sensitive studies) |
Ensure Your Electrochemical Experiments Are a Success
Choosing the right cell with the correct aperture configuration is critical for obtaining reliable data. Whether you need a standard non-sealed cell for routine analysis or a sealed cell for air-sensitive research, KINTEK has the expertise and equipment to support your work.
We provide:
- Standard & Custom Electrochemical Cells: Tailored to your specific electrode and gas management requirements.
- High-Quality Lab Equipment: Durable and precise components for consistent results.
- Expert Support: Our team understands laboratory needs and can help you select the perfect setup.
Don't let an incompatible cell compromise your research. Let KINTEK, your partner in laboratory equipment and consumables, provide the right solution for your electrochemical analysis.
Contact our experts today to discuss your specific cell requirements and get a quote!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Double-Layer Water Bath Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- What material is the five-port water bath electrolytic cell made of? High Borosilicate Glass & PTFE Explained
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments



















