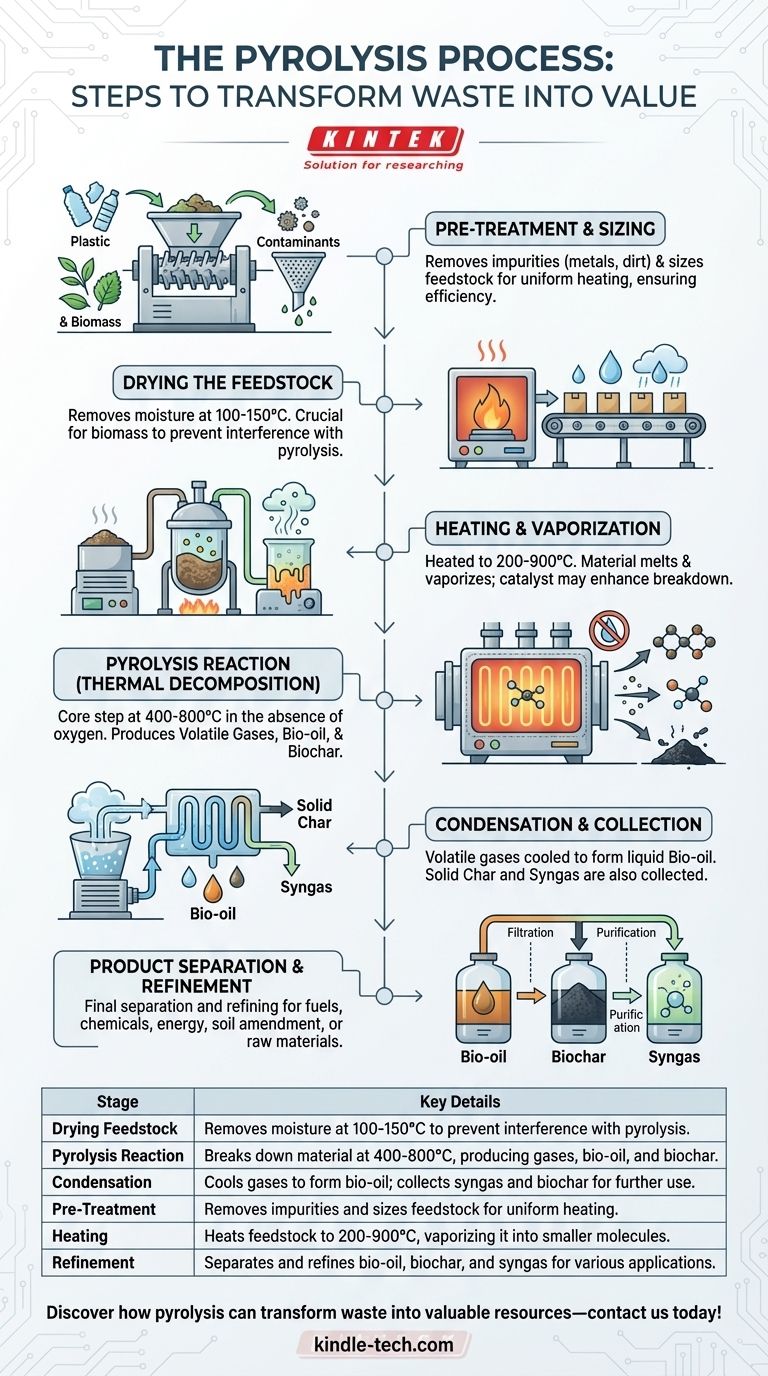
The pyrolysis process is a thermochemical decomposition of organic materials at elevated temperatures in the absence of oxygen, leading to the production of bio-oil, syngas, and biochar. The process typically involves three main stages: drying the feedstock to remove moisture, pyrolyzing the dried material at high temperatures (400-800°C) to break it down into volatile gases, liquid products, and solid char, and finally condensing and collecting the products for further use. The process can be applied to various feedstocks, including biomass and plastic waste, and involves pre-treatment, heating, and refining steps to ensure efficient conversion and recovery of usable byproducts.

Key Points Explained:
-
Drying the Feedstock
- The first step in pyrolysis is drying the feedstock to remove moisture. This is crucial because moisture can interfere with the pyrolysis reaction and reduce the efficiency of the process.
- Drying is typically done at lower temperatures (100-150°C) to ensure that the feedstock is free from water without initiating the pyrolysis reaction prematurely.
- This step is particularly important for biomass, which often contains significant moisture content.
-
Pyrolysis Reaction
- The dried feedstock is then subjected to high temperatures (400-800°C) in the absence of oxygen. This step is the core of the pyrolysis process, where the organic material undergoes thermal decomposition.
- The absence of oxygen prevents combustion and instead leads to the breakdown of the material into smaller molecules.
- The products of this stage include:
- Volatile Gases: These are primarily composed of hydrogen, carbon monoxide, carbon dioxide, and methane.
- Liquid Products (Bio-oil): A mixture of water and organic compounds, which can be further refined into fuels or chemicals.
- Solid Char (Biochar): A carbon-rich solid residue that can be used as a soil amendment or for carbon sequestration.
-
Condensation and Collection
- After the pyrolysis reaction, the volatile gases are condensed into liquid bio-oil. This is typically done by cooling the vapor phase products, causing them to condense into a liquid form.
- The solid char is collected separately, and the remaining non-condensable gases (syngas) are often captured and used as a source of energy to sustain the pyrolysis process or for other applications.
- The bio-oil can be further refined to remove impurities and improve its quality for use as a fuel or chemical feedstock.
-
Pre-Treatment and Sizing
- For materials like plastic waste, pre-treatment is necessary to remove impurities such as metals, dirt, or other contaminants. This ensures that the pyrolysis process is efficient and that the resulting products are of high quality.
- The feedstock is also ground or shredded to the required size, which facilitates uniform heating and improves the overall efficiency of the pyrolysis process.
-
Heating and Vaporization
- The feedstock is loaded into a pyrolysis reactor, where it is heated to a temperature range of 200-900°C, depending on the type of material and the desired end products.
- As the material heats up, it melts and vaporizes, breaking down into smaller molecules. This step is critical for the formation of the volatile gases and liquid products.
- The use of a catalyst may be employed to enhance the breakdown of the material and improve the yield of desired products.
-
Product Separation and Refinement
- The final step involves the separation and refinement of the pyrolysis products. The liquid bio-oil is collected and may undergo further refining to remove impurities and improve its stability and usability.
- The solid char and syngas are also collected and can be used for various applications, such as energy production, soil amendment, or as raw materials for chemical synthesis.
- The efficiency of this step is crucial for maximizing the economic and environmental benefits of the pyrolysis process.
In summary, the pyrolysis process is a multi-stage operation that involves drying, thermal decomposition, and product collection. Each step is carefully controlled to ensure the efficient conversion of organic materials into valuable byproducts, making pyrolysis a versatile and sustainable method for waste management and resource recovery.
Summary Table:
| Stage | Key Details |
|---|---|
| Drying Feedstock | Removes moisture at 100-150°C to prevent interference with pyrolysis. |
| Pyrolysis Reaction | Breaks down material at 400-800°C, producing gases, bio-oil, and biochar. |
| Condensation | Cools gases to form bio-oil; collects syngas and biochar for further use. |
| Pre-Treatment | Removes impurities and sizes feedstock for uniform heating. |
| Heating | Heats feedstock to 200-900°C, vaporizing it into smaller molecules. |
| Refinement | Separates and refines bio-oil, biochar, and syngas for various applications. |
Discover how pyrolysis can transform waste into valuable resources—contact us today!
Visual Guide
Related Products
- Electric Rotary Kiln Small Rotary Furnace Biomass Pyrolysis Plant
- Electric Rotary Kiln Continuous Working Small Rotary Furnace Heating Pyrolysis Plant
- Rotary Tube Furnace Split Multi Heating Zone Rotating Tube Furnace
- Vacuum Sealed Continuous Working Rotary Tube Furnace Rotating Tube Furnace
- 600T Vacuum Induction Hot Press Furnace for Heat Treat and Sintering
People Also Ask
- Is pyrolysis of plastic sustainable? A Balanced Look at the Environmental Trade-offs
- What temperature does a rotary kiln get to? A Guide to Process-Specific Thermal Ranges
- What are the advantages of pyrolysis of plastic? Turn Waste into Fuel and New Plastics
- What are disadvantages of pyrolysis process? Key Challenges in Energy, Cost, and Product Stability
- What is the function of a rotary kiln? A Guide to Industrial Thermal Processing
- How to regenerate activated carbon? Master the 3-Stage Thermal Process for Cost Savings
- What are the problems of rotary kiln of cement and their remedies? Achieve Long-Term Reliability and Efficiency
- What is the process of pyrolysis for making biochar? Control Temperature and Feedstock for Optimal Results



















