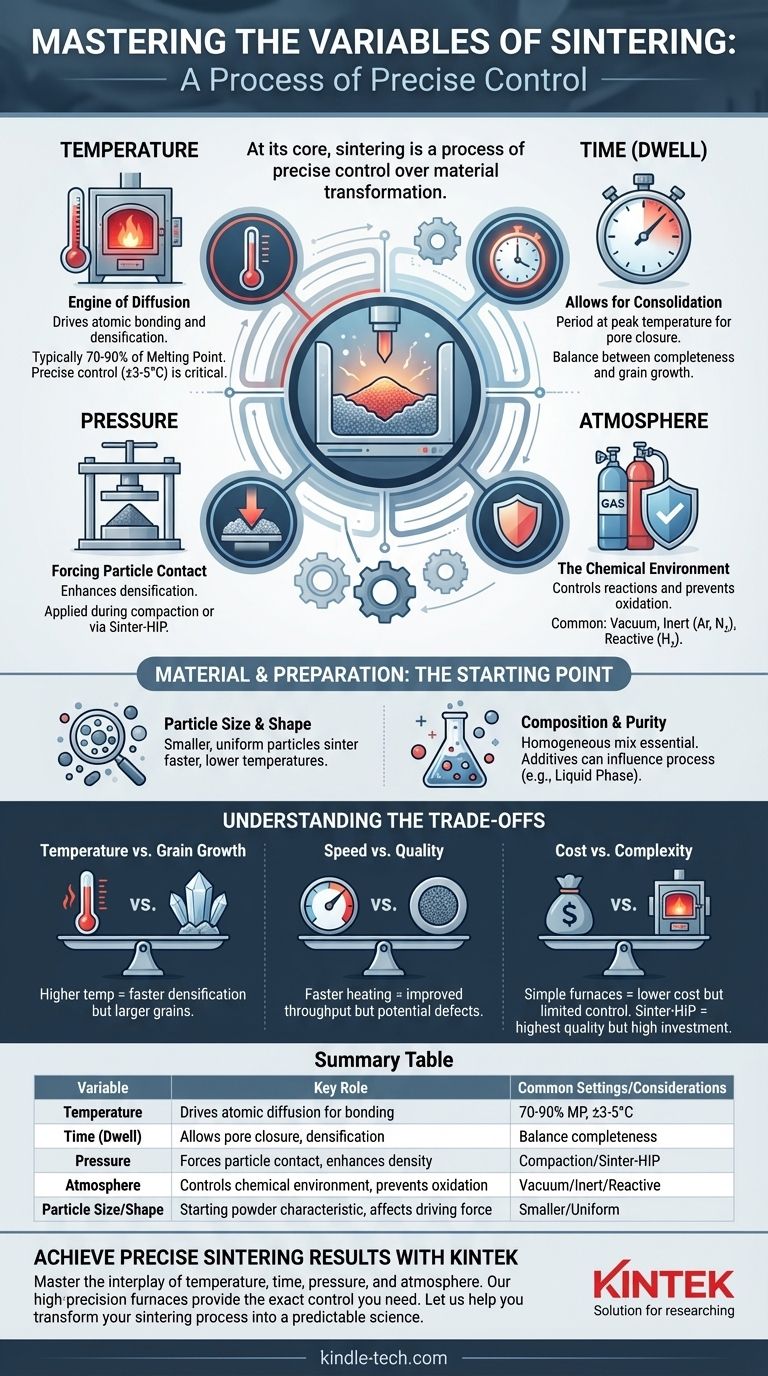
At its core, sintering is a process of precise control over material transformation. The primary variables you can manipulate are temperature, time, pressure, and atmosphere. These process parameters act upon the foundational characteristics of your material—namely its particle size and composition—to determine the final properties of the sintered part.
Sintering is not merely a heating process. It is a carefully orchestrated interplay of thermal energy, mechanical force, and chemical environment designed to control atomic diffusion, eliminate porosity, and achieve the desired density and strength in a final component.

The Core Variables of Sintering Control
Understanding each variable's specific role is crucial for moving from inconsistent results to predictable, high-quality manufacturing. These are the primary levers you use to guide the process.
Temperature: The Engine of Diffusion
Temperature is the most critical variable. It provides the thermal energy required for atoms to move across particle boundaries, enabling the necking, bonding, and densification that define the sintering process.
The correct temperature activates the material's crystalline microstructures without melting it. Sintering occurs at a specific range, typically below the material's melting point, where atomic mobility is high enough for consolidation.
Achieving and maintaining this temperature is paramount. For many high-performance applications, the furnace temperature must be controlled with extreme precision, often within a tolerance of just ±3°C to ±5°C.
Time: Allowing for Consolidation
Sintering time, often called "dwell time," is the period the material is held at the peak sintering temperature. This variable is just as important as the temperature itself.
Reaching the target temperature is not enough; the material needs sufficient time for the diffusion process to progress. This allows pores to shrink and close, leading to a denser, stronger final part. Shorter times can leave the process incomplete, while excessively long times can lead to undesirable grain growth.
Pressure: Forcing Particle Contact
Pressure enhances densification by mechanically forcing particles together. It can be applied before sintering (compaction) or during the heating cycle itself.
During initial compaction, pressure is used to form the "green" part, creating intimate contact between powder particles. Advanced techniques like Sinter-HIP (Hot Isostatic Pressing) apply high-pressure inert gas during the thermal cycle to collapse any remaining internal porosity, achieving near-full density.
Atmosphere: The Chemical Environment
The atmosphere inside the furnace plays a critical protective and sometimes reactive role. Sintering in ambient air is rare for metals, as it would cause catastrophic oxidation.
Common atmospheres include:
- Vacuum: Removes air and other gases that could react with the hot material, preventing oxidation.
- Inert Gas (Argon, Nitrogen): Creates a neutral environment that prevents unwanted chemical reactions.
- Reactive Gas (Hydrogen): Can actively remove surface oxides from metal particles, creating a cleaner surface that promotes better bonding.
Material and Preparation: The Starting Point
The success of any sintering operation is predetermined by the quality and characteristics of the starting powder.
Particle Size and Shape
Smaller particles possess higher surface energy, which provides a stronger driving force for sintering. This means they can often be sintered faster and at lower temperatures compared to larger particles.
A uniform distribution of particle sizes is also crucial for achieving consistent packing and minimizing large voids in the green compact.
Composition and Purity
The chemical makeup of the powder is fundamental. For alloys, ensuring a completely homogeneous mix of the constituent powders is essential for uniform properties in the final part.
Additives can also be used to influence the process. For example, in Liquid Phase Sintering (LPS), a small amount of a secondary material with a lower melting point is added. This material melts during heating, and the resulting liquid accelerates the densification of the primary solid particles.
Understanding the Trade-offs
Optimizing the sintering process always involves balancing competing factors. There is no single "best" setting, only the best setting for a specific goal.
Temperature vs. Grain Growth
While higher temperatures accelerate densification, they also promote grain growth. Overly large grains can reduce the material's mechanical strength and toughness. The goal is to find the temperature that maximizes density while keeping the grain size within an acceptable range.
Speed vs. Quality
Increasing the heating rate and shortening the dwell time can dramatically improve throughput and reduce energy costs. However, moving too quickly can introduce thermal stresses or trap gas in pores, leading to lower density and inferior part quality.
Cost vs. Complexity
Simple atmospheric furnaces are the least expensive option but offer limited control. Vacuum and controlled-atmosphere furnaces provide superior protection against oxidation but come at a higher capital and operational cost. Sinter-HIP systems produce the highest quality parts but represent a significant investment in equipment and complexity.
Making the Right Choice for Your Goal
Your approach to sintering should be dictated by the required performance of the final component. By understanding these variables, you can tailor the process to your specific objective.
- If your primary focus is maximum density and mechanical performance: You must prioritize precise temperature control and a protective atmosphere, likely using advanced processes like vacuum sintering or Sinter-HIP.
- If your primary focus is cost-efficiency for non-critical parts: A conventional sintering cycle with well-controlled powder characteristics and an optimized time-temperature profile is likely the most effective path.
- If your primary focus is processing novel or temperature-sensitive materials: Leveraging smaller particle sizes and pressure-assisted techniques will be key to achieving densification at lower, less damaging temperatures.
Mastering these variables transforms sintering from an art into a predictable and powerful engineering science.
Summary Table:
| Variable | Key Role | Common Settings/Considerations |
|---|---|---|
| Temperature | Drives atomic diffusion for bonding | Typically 70-90% of melting point; precise control (±3-5°C) is critical |
| Time (Dwell) | Allows for pore closure and densification | Balance between completeness and avoiding grain growth |
| Pressure | Forces particle contact; enhances density | Applied during compaction or via Sinter-HIP for near-full density |
| Atmosphere | Controls chemical environment; prevents oxidation | Vacuum, inert gas (Argon, Nitrogen), or reactive gas (Hydrogen) |
| Particle Size/Shape | Starting powder characteristic; affects driving force | Smaller, uniform particles sinter faster and at lower temperatures |
Achieve Precise Sintering Results with KINTEK
Mastering the interplay of temperature, time, pressure, and atmosphere is essential for producing high-density, high-strength sintered components. Whether you are working with metals, ceramics, or advanced alloys, the right laboratory equipment is the foundation of your success.
KINTEK specializes in lab equipment and consumables, serving laboratory needs. Our range of high-precision sintering furnaces, including vacuum and controlled-atmosphere models, provides the exact control you need to optimize these critical variables for your specific materials and application goals.
Let us help you transform your sintering process from an art into a predictable science.
Contact us today to discuss your sintering challenges and discover how our solutions can enhance your results, improve consistency, and drive your innovations forward.
Visual Guide
Related Products
- Vacuum Heat Treat and Sintering Furnace with 9MPa Air Pressure
- 1700℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- 1400℃ Muffle Oven Furnace for Laboratory
People Also Ask
- What are the main advantages of vacuum sintering? Achieve Superior Purity and Performance
- What temperature does tungsten carbide sinter at? Master the 1350°C-1500°C Liquid-Phase Sintering Process
- What are the advantages of using a vacuum hot pressing sintering furnace? Superior Density for Nanocrystalline Fe3Al
- What role does a vacuum hot pressing sintering furnace play for nanocopper? Achieve Maximum Densification Today
- How does a vacuum environment system contribute to the hot pressing sintering of B4C-CeB6? Unlock Peak Ceramic Density



















