At its core, high pressure in a reactor is most often caused by three factors: the generation of more gas molecules than were consumed during a reaction, the thermal expansion of liquids and gases as temperature rises, or a physical blockage preventing pressure from escaping the vessel. Understanding these root causes is fundamental to both process control and operational safety.
The critical insight is that high pressure is rarely a single failure point. It is almost always a result of the interplay between reaction chemistry (what's being made), thermodynamics (how energy is affecting the system), and the physical constraints of the reactor itself.

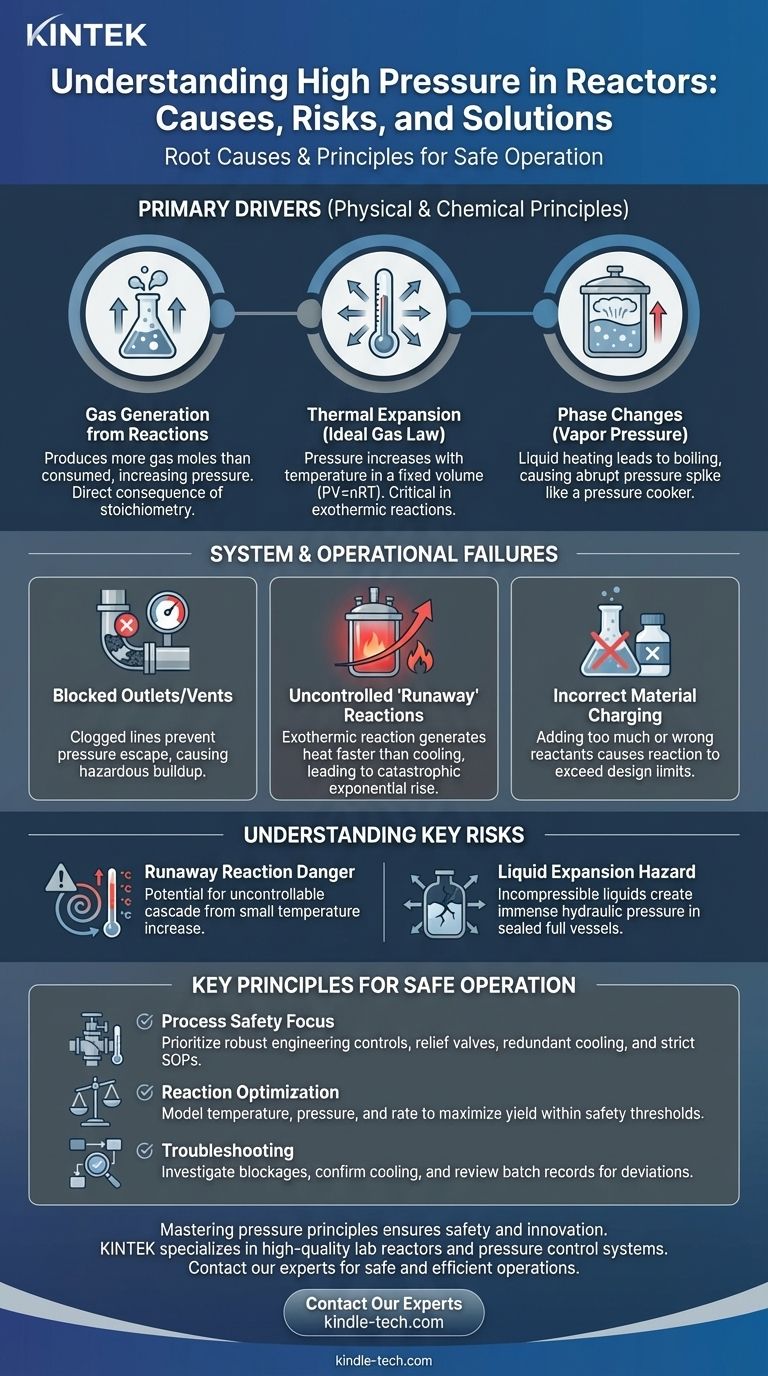
The Primary Drivers of Pressure Generation
To control pressure, you must first understand the fundamental physical and chemical principles that create it. These drivers are inherent to the process running inside the sealed vessel.
Cause 1: Gas Generation from Chemical Reactions
Many chemical reactions produce gaseous byproducts. If a reaction produces more moles of gas than it consumes, the pressure inside the sealed reactor will inevitably increase.
This is a direct consequence of stoichiometry, where the balanced chemical equation dictates the ratio of reactants to products.
Cause 2: Thermal Expansion (The Ideal Gas Law)
The relationship between pressure, volume, and temperature is governed by the Ideal Gas Law (PV=nRT). In a fixed-volume reactor, as the temperature (T) increases, the pressure (P) must also increase proportionally.
This is especially true for exothermic reactions, which release heat, raising the system's temperature and, consequently, its pressure.
Cause 3: Phase Changes (Vapor Pressure)
Heating a liquid in a sealed container increases its vapor pressure. If the temperature exceeds the liquid's boiling point at a given pressure, it will begin to boil, rapidly generating a large volume of gas.
This phase change can cause an extremely abrupt and dangerous spike in pressure, similar to how a pressure cooker operates.
System and Operational Failures
Beyond the core chemistry and physics, high-pressure events are often triggered or worsened by failures in the reactor system or human error during operation.
Cause 4: Blocked Outlets or Vents
A reactor is a system designed for flow. If an outlet line, vent, or pressure relief valve becomes clogged or is inadvertently closed, the normal escape path for pressure is cut off.
Even a slow, gas-producing reaction can quickly become hazardous if the system has no way to vent the accumulating pressure.
Cause 5: Uncontrolled "Runaway" Reactions
This is one of the most severe hazards in chemical processing. A runaway occurs when an exothermic reaction generates heat faster than the cooling system can remove it.
This creates a dangerous feedback loop: more heat increases the reaction rate, which generates even more heat, leading to an exponential and often catastrophic rise in both temperature and pressure.
Cause 6: Incorrect Material Charging
Adding too much reactant, the wrong concentration of a catalyst, or forgetting a crucial inhibitor can cause a reaction to proceed much faster or more energetically than designed. This deviation from the established procedure can easily overwhelm the system's ability to control temperature and pressure.
Understanding the Key Risks
Recognizing the causes is only half the battle. Understanding the specific risks associated with them is crucial for preventing accidents.
The Danger of Exothermic Reactions
The primary risk with heat-releasing reactions is the potential for a runaway. The danger lies in the feedback loop, where a small initial increase in temperature can cascade into an uncontrollable event if not managed by a robust cooling system.
The Incompressibility of Liquids
While we often focus on gases, the thermal expansion of liquids is a significant and often underestimated hazard. Because liquids are nearly incompressible, even a small increase in temperature in a completely full, sealed vessel can generate immense hydraulic pressure, easily capable of rupturing the reactor.
The Fallacy of "Slow" Reactions
A common mistake is assuming a reaction that is slow at ambient temperature will remain manageable when heated. Reaction rates can increase exponentially with temperature, turning a slow, controlled process into a violent, high-pressure event with only a modest increase in heat.
Key Principles for Safe Reactor Operation
Your approach to managing reactor pressure should be dictated by your primary objective, whether it's ensuring safety, optimizing a process, or troubleshooting a problem.
- If your primary focus is process safety: Your priority must be robust engineering controls like properly sized pressure relief valves, redundant cooling systems, and strict adherence to standard operating procedures (SOPs).
- If your primary focus is reaction optimization: You must precisely model and understand the relationship between temperature, pressure, and reaction rate to maximize yield without crossing established safety thresholds.
- If your primary focus is troubleshooting a high-pressure event: Systematically investigate for outlet blockages, confirm the cooling system is fully operational, and meticulously review recent batch records for any deviations from the plan.
Mastering the principles behind pressure generation transforms a reactor from an unpredictable risk into a controlled and powerful tool for innovation.
Summary Table:
| Cause Category | Specific Cause | Primary Risk |
|---|---|---|
| Physical & Chemical Drivers | Gas Generation from Reactions | Pressure buildup from gas moles produced |
| Thermal Expansion (Ideal Gas Law) | Pressure increase with temperature in a fixed volume | |
| Phase Changes (Vapor Pressure) | Rapid pressure spike from boiling liquids | |
| System & Operational Failures | Blocked Outlets or Vents | No escape path for pressure, leading to overpressure |
| Uncontrolled Runaway Reactions | Exponential heat and pressure increase | |
| Incorrect Material Charging | Reaction proceeds faster/more energetically than designed |
Ensure your reactor operations are safe and efficient. High-pressure events are a major risk, but proper equipment and expert support make them manageable. KINTEK specializes in high-quality lab reactors, pressure control systems, and consumables designed for demanding laboratory environments. Our team can help you select the right equipment and develop safe operating protocols. Contact our experts today to discuss your specific reactor needs and enhance your lab's safety and performance.
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Customizable High Pressure Reactors for Advanced Scientific and Industrial Applications
- Mini SS High Pressure Autoclave Reactor for Laboratory Use
- Stainless High Pressure Autoclave Reactor Laboratory Pressure Reactor
- High Pressure Laboratory Autoclave Reactor for Hydrothermal Synthesis
People Also Ask
- Why are high-precision pressure sensors and temperature control systems critical for hydrothermal reaction equilibrium?
- Why use high-pressure reactors for food waste pretreatment? Boost Hydrogen Production Efficiency Today!
- What is the function of a PTFE-lined hydrothermal autoclave in cys-CDs synthesis? Achieve High-Purity Carbon Dots
- What role does a PTFE-lined stainless steel autoclave play in the synthesis of BiOBr precursor nanosheets?
- What are the technical characteristics of PTFE (Teflon) lined hydrothermal reactors? Comparing α-ZrP Synthesis Methods



















