To properly prepare for an experiment, the PTFE electrode stand, along with its clamps and fixtures, must be thoroughly wiped down. The primary cleaning agents are deionized water or a suitable alcohol, such as ethanol. This procedure is essential for removing any residual electrolytes, grease, dust, or other impurities that could otherwise contaminate your system and compromise the integrity of your results.
The core purpose of cleaning the electrode stand is not merely cosmetic. It is a critical step to ensure experimental reproducibility by eliminating chemical and physical contaminants that can introduce artifacts and invalidate your electrochemical measurements.

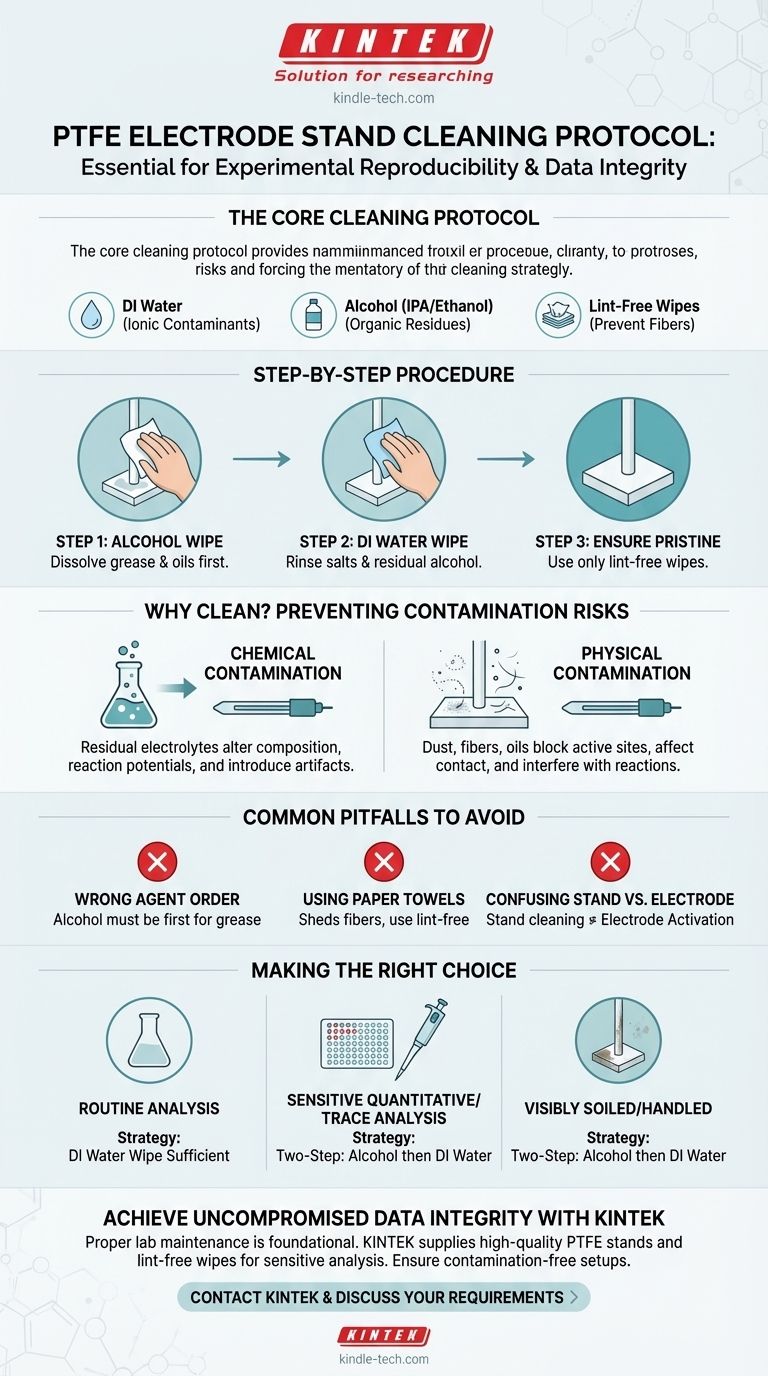
The Core Cleaning Protocol
A pristine experimental setup begins with a meticulous cleaning procedure. While PTFE is an inert material, its surface can harbor contaminants that interfere with sensitive measurements.
Required Materials
The materials for this task are simple and should be readily available in any electrochemical lab. You will need:
- Deionized (DI) Water: For rinsing away ionic contaminants.
- Alcohol: Isopropyl alcohol (IPA) or ethanol are standard choices for dissolving organic residues.
- Lint-Free Wipes: Essential for cleaning without leaving behind fiber particles.
The Step-by-Step Procedure
The process is straightforward. First, moisten a lint-free wipe with your chosen cleaning agent. Systematically wipe down all surfaces of the stand, paying close attention to the electrode clamps and any other fixtures that will be near the electrolytic cell. If using both alcohol and water, clean with alcohol first to remove grease, then follow with a separate deionized water wipe to remove any residual salts or alcohol.
Why Use These Specific Agents?
The choice of cleaning agent is deliberate. Deionized water is highly effective at dissolving and removing residual salts or electrolytes from previous experiments. Alcohol is a solvent for non-polar contaminants like grease and oils from handling. Using them ensures you address both major classes of potential impurities.
The Principle: Preventing System Contamination
Failing to clean the stand properly introduces uncontrolled variables into your experiment. This oversight is a common source of error that can lead to inaccurate data and flawed conclusions.
The Risk of Surface Contaminants
While the PTFE stand itself is chemically resistant, any substance adhering to its surface can leach into your electrolyte. This can alter the electrolyte's composition, introduce competing electrochemical reactions, or poison the surface of your working electrode.
Chemical Contamination
The most common chemical contaminants are residues from prior experiments. Even a trace amount of a different electrolyte can dramatically shift reaction potentials or introduce unexpected peaks in techniques like cyclic voltammetry.
Physical Contamination
Dust, fibers from paper towels, or oils from fingerprints are forms of physical contamination. These can block active sites on the electrode surface, affect electrical contact in the clamps, or introduce organic compounds that interfere with your reaction of interest.
Common Pitfalls to Avoid
Executing the cleaning protocol correctly is as important as performing it at all. Small mistakes can undermine the entire effort.
Choosing Between Deionized Water and Alcohol
Do not treat them as interchangeable. If you suspect grease or oil contamination, always start with alcohol. If you are simply running a similar experiment and need to remove old electrolyte, deionized water is often sufficient. For maximum certainty, use both.
The Importance of Lint-Free Wipes
Using standard paper towels or cloths is a frequent mistake. These materials shed fibers that become physical contaminants in your cell, potentially ruining sensitive measurements. Always use wipes specifically designed for cleanroom or laboratory environments.
Distinguishing Stand Cleaning from Electrode Activation
It is critical to understand that cleaning the stand is different from preparing the electrode. Cleaning the stand removes external contaminants. Activating the electrode, often done via pre-electrolysis or polishing, is a separate step meant to remove surface oxides and create a pristine, repeatable electrode surface for the reaction.
Making the Right Choice for Your Experiment
Your cleaning strategy should match the sensitivity of your work.
- If your primary focus is routine or qualitative analysis: A thorough wipe-down with deionized water to remove previous electrolyte is typically sufficient.
- If you are conducting sensitive quantitative or trace analysis: Employ a two-step process using alcohol first, followed by deionized water, to eliminate all potential organic and ionic contaminants.
- If the stand is visibly soiled or has been handled extensively: Always begin with an alcohol wipe to dissolve organic residues before the final deionized water rinse.
Ultimately, this simple cleaning step is a foundational investment in the accuracy and reliability of your experimental data.
Summary Table:
| Cleaning Step | Purpose | Key Consideration |
|---|---|---|
| Wipe with Alcohol (IPA/Ethanol) | Dissolves and removes organic residues, grease, and oils. | Use first if contamination is suspected or for sensitive work. |
| Wipe with Deionized (DI) Water | Rinses away ionic contaminants and residual salts/electrolytes. | Follows alcohol wipe or is sufficient for routine cleaning. |
| Use Lint-Free Wipes | Prevents introduction of fiber particles as physical contaminants. | Avoid paper towels or cloths that shed fibers. |
Achieve Uncompromised Data Integrity with KINTEK
Proper lab equipment maintenance is the foundation of reliable science. The meticulous cleaning protocol outlined here is just one example of the detailed preparation required for accurate electrochemical analysis.
At KINTEK, we specialize in supplying the high-quality lab equipment and consumables—like PTFE electrode stands and lint-free wipes—that your research depends on. Our products are designed to meet the rigorous demands of sensitive quantitative and trace analysis, helping you eliminate variables and focus on discovery.
Ready to enhance your lab's precision and efficiency? Let our experts help you select the right tools for your specific experimental needs.
Contact KINTEK today via our form to discuss your laboratory requirements and ensure your setups are contamination-free from the start.
Visual Guide
Related Products
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Platinum Sheet Electrode for Laboratory and Industrial Applications
- Electrolytic Electrochemical Cell for Coating Evaluation
- Custom PTFE Teflon Parts Manufacturer PTFE Beaker and Lids
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What is the precaution regarding temperature when using an all-PTFE electrolytic cell? Essential Thermal Safety Tips
- What are the standard opening specifications for all-PTFE electrolytic cells? A Guide to Sealed vs. Non-Sealed Ports
- What are the typical volumes for an all-PTFE electrolytic cell? Choose the Right Size for Your Experiment
- How should an all-PTFE electrolytic cell be handled to prevent mechanical damage? Protect Your Investment and Data Integrity
- What is the proper cleaning method for an all-PTFE electrolytic cell? Essential Tips for Surface Integrity