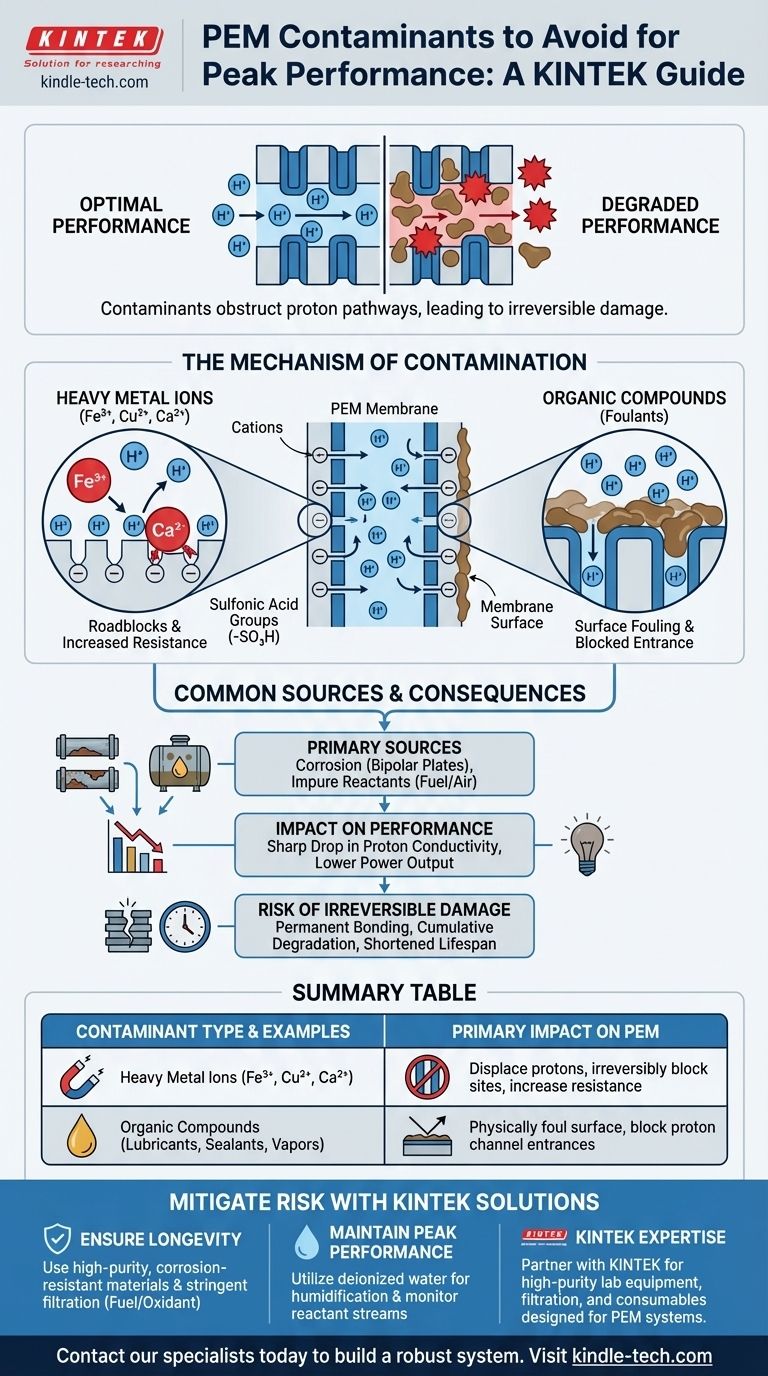
To ensure optimal performance and longevity, a proton exchange membrane (PEM) must be rigorously protected from two primary categories of contaminants: heavy metal ions and various organic compounds. These substances compromise the membrane's core function by attaching to its active sites, which obstructs the pathways for protons and leads to a significant and often irreversible degradation of performance.
The central challenge is that the membrane's functional groups, which are designed to transport protons, have a stronger chemical affinity for contaminants like metal ions. This causes contaminants to displace protons and physically block the membrane, fundamentally crippling the system's efficiency and lifespan.

The Mechanism of Contamination: How Performance Degrades
Understanding how contaminants interact with the membrane at a molecular level is crucial for preventing system failure. The entire process hinges on the specialized chemistry of the membrane itself.
The Role of Sulfonic Acid Groups
The PEM works because it is embedded with sulfonic acid groups (-SO₃H). These fixed, negatively charged sites are the "proton highways," allowing positively charged hydrogen ions (protons) to hop from one site to the next across the membrane.
Contamination by Heavy Metal Ions
Heavy metal cations, such as iron (Fe³⁺), copper (Cu²⁺), or calcium (Ca²⁺), are highly detrimental. Because of their higher positive charge, they are more strongly attracted to the negative sulfonic acid sites than a single proton (H⁺).
When these ions enter the system, they displace the protons and bind tightly to the sulfonic groups. This effectively creates a roadblock, reducing the number of available pathways for proton transport and increasing the membrane's electrical resistance.
Fouling by Organic Compounds
Organic compounds present a different, but equally damaging, threat. They act as foulants, physically adsorbing onto the membrane surface.
This creates a non-conductive layer that can block the entrance to the proton channels. This fouling prevents protons from even beginning their journey across the membrane, severely limiting the system's power output.
Common Sources and Consequences
Contaminants are not abstract threats; they originate from specific sources within the operating environment and have tangible, negative consequences on the system.
Primary Contaminant Sources
Contamination almost always stems from the balance-of-plant components or the reactant streams. Corrosion from metallic bipolar plates, piping, or fittings can release metal ions into the system.
Likewise, impurities in the hydrogen fuel or airborne organic vapors from lubricants, seals, or even ambient air pollution can be introduced through the air stream.
The Impact on Performance
The immediate effect of contamination is a sharp drop in proton conductivity. This directly translates to lower cell voltage and a reduction in overall power output.
The Risk of Irreversible Damage
Crucially, this damage is often permanent. Once a metallic ion has bonded to a sulfonic acid site, it is extremely difficult to remove. This leads to a cumulative degradation that shortens the operational lifespan of the entire fuel cell or electrolyzer stack.
How to Mitigate Contamination Risk
Preventing contaminants from reaching the membrane is the only effective strategy. Your approach should be based on controlling the purity of every element that interacts with the PEM.
- If your primary focus is system longevity: Prioritize the use of high-purity, corrosion-resistant materials for all system components and implement stringent filtration for both fuel and oxidant streams.
- If your primary focus is maintaining peak performance: Ensure the use of high-purity deionized water for humidification and consider regular monitoring of reactant streams for potential impurities.
Proactive contamination control is the cornerstone of reliable and long-lasting PEM system operation.
Summary Table:
| Contaminant Type | Common Examples | Primary Impact on PEM |
|---|---|---|
| Heavy Metal Ions | Iron (Fe³⁺), Copper (Cu²⁺), Calcium (Ca²⁺) | Displace protons, irreversibly block sulfonic acid sites, increase resistance |
| Organic Compounds | Lubricants, sealants, airborne vapors | Physically foul membrane surface, block proton channel entrances |
Ensure the longevity and peak performance of your PEM system with KINTEK.
Controlling contamination is critical for reliable operation. KINTEK specializes in high-purity lab equipment and consumables, including corrosion-resistant components and filtration solutions designed to protect sensitive systems like proton exchange membranes. Our products help you maintain the purity of fuel, oxidant, and humidification streams, directly addressing the contamination risks outlined in this article.
Let our expertise support your research and development. Contact our specialists today to discuss how we can help you build a more robust and efficient PEM system.
Visual Guide
Related Products
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Platinum Auxiliary Electrode for Laboratory Use
- Electrode Fixture for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
People Also Ask
- What is an electrolysis cell also known as? Understanding Electrolytic vs. Galvanic Cells
- How do specialized electrolytic cells facilitate electrochemical testing? Enhance Stainless Steel Corrosion Analysis
- What are the proper storage procedures for the multifunctional electrolytic cell? Protect Your Investment and Ensure Data Accuracy
- What is a proton exchange membrane? The Selective Heart of Hydrogen Energy Systems
- What general precaution should be taken when handling the electrolytic cell? Ensure Safe and Accurate Lab Results










