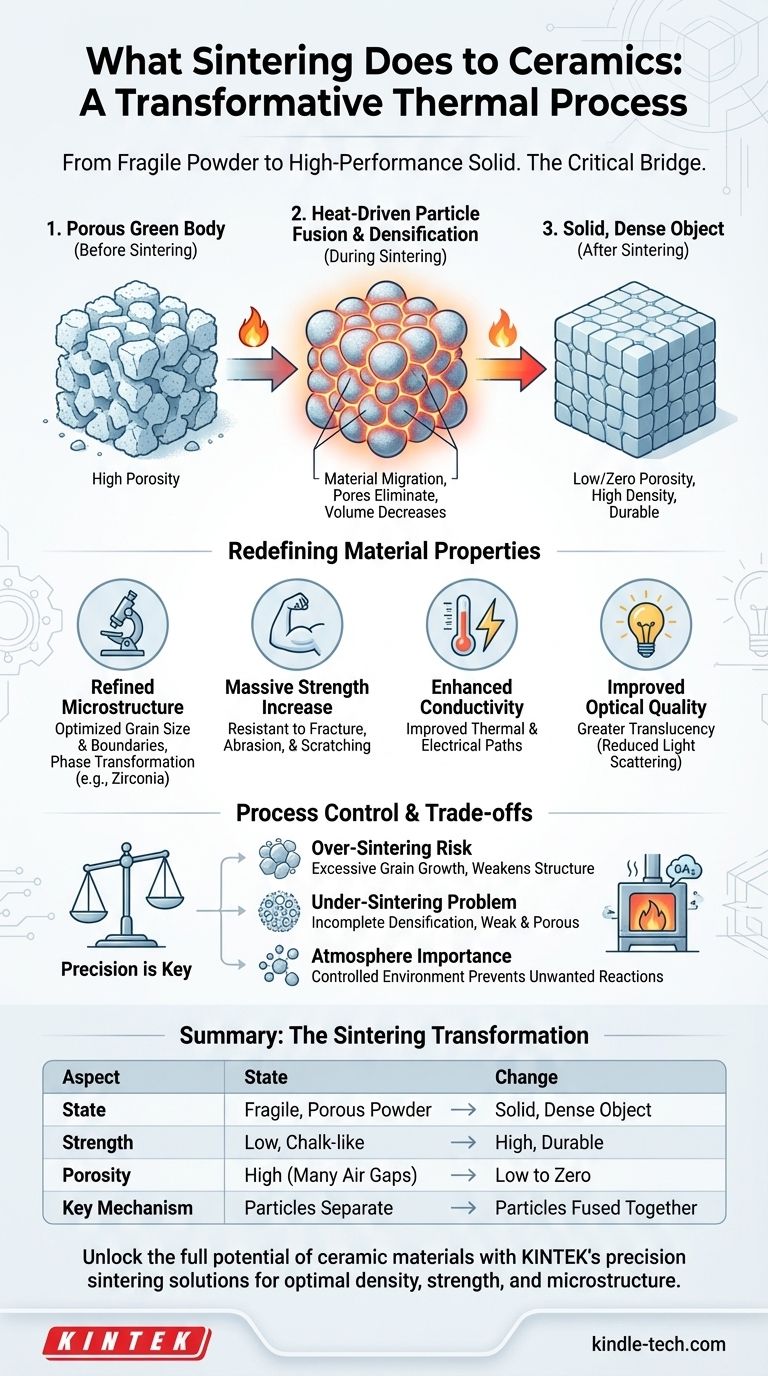
In essence, sintering is a transformative thermal process that converts a compacted ceramic powder into a solid, dense object. By heating the material to a high temperature below its melting point, individual particles fuse together, eliminating the pores between them and drastically increasing the material's density, strength, and overall durability.
The true purpose of sintering is to convert a fragile, porous compact of ceramic powder into a dense, solid body. This is achieved by using high heat to fuse individual particles together, fundamentally altering the material's microstructure to unlock its final, high-performance properties.

The Fundamental Goal: From Powder to Solid
Sintering is the critical bridge between a weak powder form and a robust, functional component. The process is a carefully controlled journey of structural transformation.
The Starting Point: A Porous "Green" Body
Before sintering, ceramic powder is typically pressed or formed into a desired shape. This initial object, often called a "green body," is chalk-like, fragile, and full of tiny air gaps, or porosity.
The Mechanism: Heat-Driven Particle Fusion
When subjected to high heat, the atoms at the contact points between powder particles become highly active. This energy drives material migration, causing the particles to bond and merge.
Think of it like a pile of compacted snow on a day when the temperature hovers just below freezing. The individual snowflakes begin to fuse, and over time, the pile transforms into a solid, dense block of ice.
The Result: Densification and Shrinkage
As the particles fuse and the pores between them are eliminated, the overall volume of the ceramic body decreases. This densification is a direct measure of the process's success, resulting in a significantly stronger and less porous material.
How Sintering Redefines Material Properties
The densification achieved through sintering is not just a physical change; it is the mechanism that unlocks the characteristic high-performance properties of engineered ceramics.
A Shift in Microstructure
At a microscopic level, sintering refines the material's internal architecture, or microstructure. It controls the final grain size, the shape of the grain boundaries, and the distribution of any remaining pores. For some materials like zirconia, it also triggers a crucial phase transformation, changing the crystal structure to a much harder and denser state.
The Impact on Mechanical Strength
The primary benefit of sintering is a massive increase in strength and durability. The pores in an unsintered body act as microscopic stress points where cracks can easily start. By eliminating these voids, sintering creates a solid, continuous structure that is far more resistant to fracture, abrasion, and scratching.
Enhancing Thermal and Electrical Properties
A dense material provides a more direct path for energy to travel. The elimination of air-filled pores, which act as insulators, improves a ceramic's ability to conduct heat and, in some cases, electricity.
Improving Optical Qualities
In certain ceramics, reducing porosity also improves translucency. Pores scatter light, making a material appear opaque. By creating a dense, uniform microstructure, light can pass through the material with less interruption, which is critical for applications like dental crowns.
Understanding the Trade-offs and Process Control
Achieving the desired properties is not automatic. Sintering is a precise science where time, temperature, and atmosphere must be perfectly optimized.
The Risk of Over-Sintering
Applying too much heat or holding the temperature for too long can cause the material's grains to grow excessively large. While the material will be dense, these large grains can paradoxically weaken the final structure, making it more brittle.
The Problem of Under-Sintering
Conversely, insufficient heat or time will result in incomplete densification. The remaining porosity will leave the ceramic weak, porous, and unable to meet its performance specifications.
The Importance of Atmosphere
The process must occur in a controlled atmosphere. The gases present in the furnace can react with the ceramic, altering its chemistry and properties. An optimized sintering process accounts for this to guarantee the final product is exactly as designed.
Making the Right Choice for Your Goal
Understanding the purpose of sintering helps you evaluate ceramic materials and troubleshoot issues.
- If your primary focus is maximum strength and durability: The key is achieving the highest possible density by eliminating porosity through a fully optimized sintering cycle.
- If you are troubleshooting a failed ceramic part: Investigate the sintering process, as incomplete densification (under-sintering) or excessive grain growth (over-sintering) are common root causes of failure.
- If you are selecting a ceramic product: The term "sintered" signifies that the material has undergone this critical densification process to become a high-performance, non-porous, and durable final product.
Ultimately, sintering is the crucial step that unlocks the true potential engineered into a ceramic material.
Summary Table:
| Aspect | Before Sintering (Green Body) | After Sintering |
|---|---|---|
| State | Fragile, porous powder compact | Solid, dense object |
| Strength | Low, chalk-like | High, durable |
| Porosity | High (many air gaps) | Low to zero |
| Key Change | Particles are separate | Particles are fused together |
Unlock the full potential of your ceramic materials with KINTEK's precision sintering solutions.
Whether you are developing new ceramic components or troubleshooting existing processes, our expertise in lab equipment and consumables ensures you achieve the perfect density, strength, and microstructure. Let us help you optimize your sintering cycle for maximum performance.
Contact our experts today to discuss your specific laboratory needs and discover the right equipment for your success.
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
People Also Ask
- How is a muffle furnace utilized to evaluate titanium-based composite materials? Master Oxidation Resistance Testing
- What is the construction of a muffle furnace? A Deep Dive into Its Core Systems
- What happens in the muffle furnace? Achieve Pure, Uniform High-Temperature Processing
- What is the temperature range of a laboratory muffle furnace? Find the Right Model for Your Application
- What is the operating temperature of the muffle furnace? Find Your Ideal Range for Lab Success



















