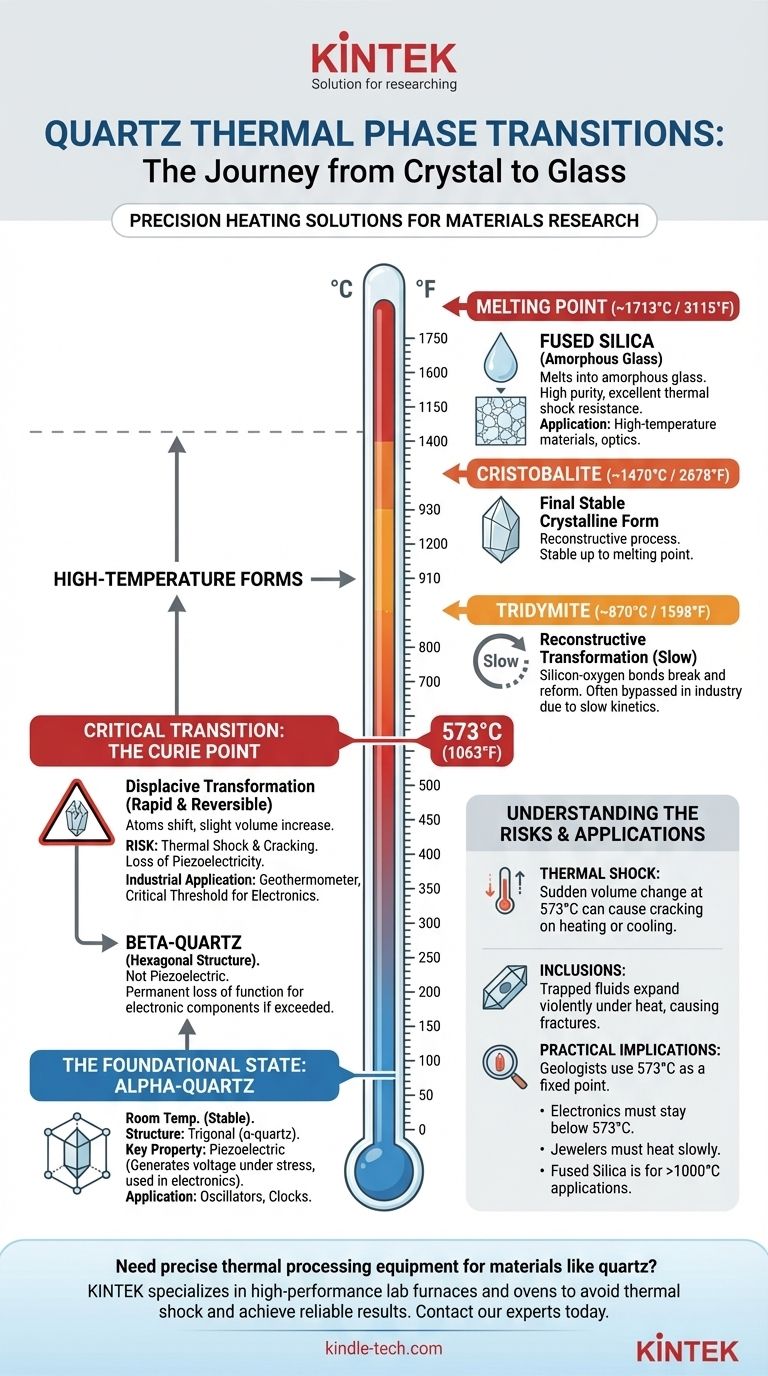
When quartz is heated, it does not simply melt. Instead, it undergoes a series of predictable phase transitions, transforming into different crystalline structures (polymorphs) at specific temperature thresholds. The most critical and immediate change occurs at 573°C (1063°F), where common alpha-quartz abruptly shifts into beta-quartz, a change that fundamentally alters its physical properties.
The behavior of quartz under heat is a journey through different structural forms, not a direct path to a liquid state. Understanding these specific transition points—especially the 573°C threshold—is the critical factor in determining its stability and usefulness in any application, from geology to electronics.

The Foundational State: Alpha-Quartz
What is Alpha-Quartz?
At room temperature and normal atmospheric pressure, all naturally occurring quartz is alpha-quartz (α-quartz).
This is the stable, low-temperature form of silicon dioxide (SiO₂). Its atoms are arranged in a trigonal crystal system.
The Piezoelectric Effect
A defining characteristic of alpha-quartz is its piezoelectric property. This means it generates a small electrical voltage when subjected to mechanical stress.
This effect is the foundation for its use in electronics, such as precise oscillators for watches, radios, and computers. This property is unique to the alpha-quartz structure.
The First Critical Transition: The Curie Point
Alpha to Beta-Quartz at 573°C (1063°F)
When heated to 573°C, alpha-quartz undergoes a rapid and reversible transformation into beta-quartz (β-quartz). This specific temperature is known as the Curie Point for quartz.
This is a displacive transformation, meaning the atoms shift their positions slightly, but the fundamental bonds in the crystal lattice are not broken. Because of this, the change happens almost instantly.
What Changes During the Transition?
The crystal structure shifts from trigonal (alpha) to hexagonal (beta). This causes a slight but sudden increase in volume.
Crucially, beta-quartz is not piezoelectric. The change in symmetry cancels out this property. If an electronic component made of quartz is heated past this point, it will permanently lose its essential function, even after cooling.
Why 573°C is a Key Figure
This sharp transition point is so reliable that it is used by geologists as a geothermometer to determine the temperatures at which certain rocks were formed. In industry, it represents a critical threshold that must be carefully managed.
Beyond Beta-Quartz: High-Temperature Forms
The Transition to Tridymite (~870°C)
As temperatures rise much higher, beta-quartz can transform into tridymite. This change begins around 870°C (1598°F).
Unlike the alpha-beta shift, this is a reconstructive transformation. It requires the breaking and reforming of silicon-oxygen bonds, making it a very slow and sluggish process. In many industrial settings, this phase is bypassed entirely due to its slow kinetics.
The Final Crystalline Form: Cristobalite (~1470°C)
At approximately 1470°C (2678°F), tridymite will reconstruct itself into the final stable crystalline form of silica: cristobalite.
This is the form of silica that is stable right up to the melting point. Like the tridymite transition, it is a slow, reconstructive process.
The Melting Point: Fused Silica (~1713°C)
Finally, at around 1713°C (3115°F), cristobalite melts. The resulting liquid, upon cooling, does not re-form a crystal structure but instead becomes an amorphous glass.
This non-crystalline material is known as fused quartz or fused silica. It has exceptionally high purity and excellent thermal shock resistance.
Understanding the Risks and Pitfalls
The Danger of Thermal Shock
The sudden volume change that occurs at the 573°C alpha-beta transition is a major point of failure.
Heating or cooling quartz too rapidly through this temperature can cause immense internal stress, leading the crystal to crack or shatter. This is the primary risk in any thermal application.
The Inversion Problem on Cooling
The transition is reversible. When beta-quartz cools below 573°C, it inverts back to alpha-quartz. If this cooling is not slow and controlled, the same cracking from volume change can occur.
This is a well-known problem in the ceramics industry, where quartz is a common component of clays and glazes.
Inclusions and Fluid Pockets
Natural quartz crystals often contain microscopic inclusions of other minerals, water, or gas.
When heated, these trapped fluids can expand dramatically, creating immense pressure from within the crystal and causing it to fracture unexpectedly, even at temperatures well below the phase transition points.
How to Apply This Knowledge
Understanding these transformations is not academic; it dictates how quartz should be handled and utilized in practice.
- If you are a geologist or material scientist: Use the 573°C alpha-beta transition as a fixed point to calibrate equipment or as a "fossil thermometer" to understand the thermal history of rocks.
- If you are working in electronics: You must ensure that any quartz oscillator component never approaches 573°C, as this will irreversibly destroy its critical piezoelectric function.
- If you are a jeweler or lapidary: Heat quartz slowly and evenly, being especially cautious around the 573°C threshold, and always inspect for internal fluid inclusions to prevent shattering.
- If you are fabricating high-temperature materials: Recognize that fused silica (melted quartz glass), not crystalline quartz, is the correct choice for applications requiring stability above 1000°C due to its lack of destructive phase transitions.
By respecting these fundamental thermal thresholds, you can harness the remarkable properties of quartz while avoiding its inherent vulnerabilities.
Summary Table:
| Temperature | Phase Transition | Key Change | Practical Implication |
|---|---|---|---|
| 573°C (1063°F) | Alpha-Quartz → Beta-Quartz | Loss of piezoelectricity; slight volume increase | Critical threshold for electronics; risk of thermal shock |
| ~870°C (1598°F) | Beta-Quartz → Tridymite | Slow reconstructive transformation | Often bypassed in industrial processes |
| ~1470°C (2678°F) | Tridymite → Cristobalite | Final stable crystalline form | Stable up to melting point |
| ~1713°C (3115°F) | Cristobalite → Fused Silica (Glass) | Melts into amorphous glass | Excellent thermal shock resistance; high purity |
Need precise thermal processing equipment for materials like quartz? KINTEK specializes in high-performance lab furnaces and ovens designed for controlled heating and cooling, helping you avoid thermal shock and achieve reliable results. Whether you're in materials science, geology, or electronics manufacturing, our solutions ensure you respect critical temperature thresholds. Contact our experts today to find the perfect equipment for your application!
Visual Guide
Related Products
- 1700℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Laboratory Rapid Thermal Processing (RTP) Quartz Tube Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
- 1700℃ Muffle Oven Furnace for Laboratory
People Also Ask
- Why is a high-purity alumina lining required for high-temperature tube furnaces? Ensure Accurate Biomass Research
- What is the function of alumina tubes and alumina wool in a pyrolysis furnace? Optimize Your Biochar Production Quality
- What is the ceramic tube high temperature? From 1100°C to 1800°C, Choose the Right Material
- How do you clean a tube furnace tube? A Step-by-Step Guide to Safe and Effective Cleaning
- What is the role of corundum tubes in oxygen permeation testing? Ensure Integrity for Bi-doped Membranes



















