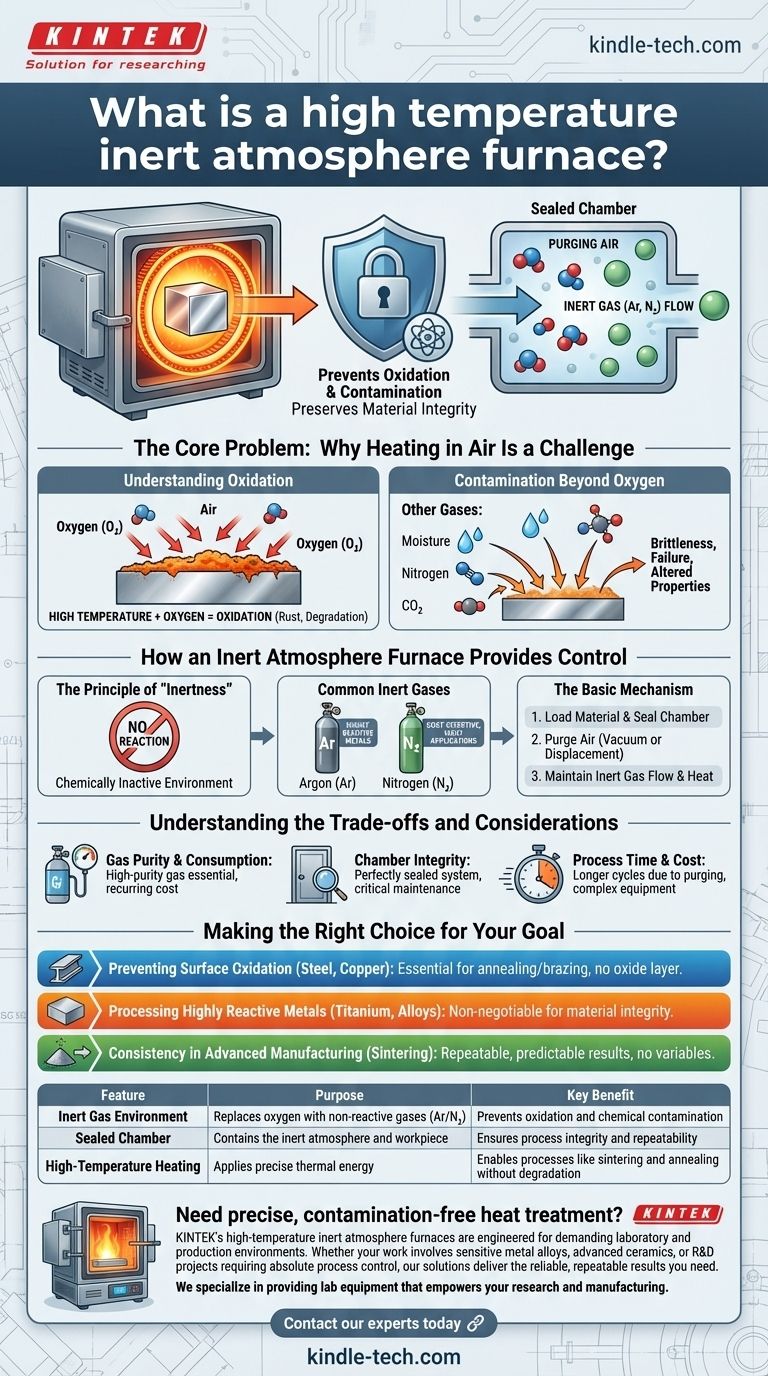
At its core, a high-temperature inert atmosphere furnace is a specialized oven designed to heat materials in an environment free of oxygen and other reactive gases. Its purpose is to perform heat-treatment processes like brazing, sintering, or annealing without causing unwanted chemical reactions, primarily oxidation. This controlled environment ensures the material's chemical composition and structural integrity are preserved.
The fundamental challenge in high-temperature processing is that heat accelerates chemical reactions, and the oxygen in our air is highly reactive. An inert atmosphere furnace solves this by replacing the air with a non-reactive gas, creating a safe, neutral environment for the material.

The Core Problem: Why Heating in Air Is a Challenge
When you need to heat materials to hundreds or even thousands of degrees, the surrounding atmosphere becomes a critical factor. Performing this in open air introduces significant, often destructive, variables.
Understanding Oxidation
At high temperatures, oxygen in the air aggressively reacts with the surface of most materials. This process is oxidation.
We see a slow version of this as rust on iron, but high heat acts as a powerful catalyst. This can weaken the material, change its electrical properties, or simply ruin its surface finish.
Contamination Beyond Oxygen
Air isn't just oxygen. It also contains moisture, nitrogen, carbon dioxide, and other trace elements.
Depending on the material and temperature, these gases can also react with the workpiece, leading to contamination, brittleness, or a failure to achieve the desired material properties.
How an Inert Atmosphere Furnace Provides Control
This type of furnace is engineered specifically to eliminate the variable of a reactive atmosphere. It gives operators precise control over the chemical environment.
The Principle of "Inertness"
The term inert means chemically inactive. The furnace creates this environment by purging the ambient air from a sealed chamber and replacing it with a gas that will not react with the material being heated.
This prevents oxidation and other unwanted reactions, isolating the process to the effects of heat alone.
Common Inert Gases
The most common gases used are Argon (Ar) and Nitrogen (N₂).
Argon is completely inert and is the go-to choice for highly reactive metals or the most sensitive processes. Nitrogen is technically less inert than Argon but is suitable for many applications and is often more cost-effective.
The Basic Mechanism
The process involves a few key steps. First, the material is placed inside a tightly sealed chamber. Second, the air is removed, either by a vacuum pump or by "purging"—flooding the chamber with the inert gas to displace the lighter air.
Finally, a steady, low-pressure flow of the inert gas is maintained while the heating elements bring the chamber to the target temperature.
Understanding the Trade-offs and Considerations
While incredibly useful, these furnaces introduce their own set of operational requirements and complexities compared to a standard oven.
Gas Purity and Consumption
The effectiveness of the process is directly tied to the purity of the inert gas. Even a small percentage of oxygen can compromise the result. This creates a recurring operational cost for high-purity gas cylinders or generators.
Chamber Integrity
The entire system relies on the chamber being perfectly sealed. Any leak, no matter how small, will allow oxygen to seep in, defeating the purpose of the inert atmosphere. Regular maintenance and seal checks are critical.
Process Time and Cost
Purging the chamber of air takes time, adding to the overall cycle time of a heat treatment. The equipment itself is also more complex and expensive than a standard furnace due to the need for sealed chambers, gas handling systems, and precise controls.
Making the Right Choice for Your Goal
Using an inert atmosphere is not just a feature; it's a solution to specific material processing problems. The decision to use one should be tied directly to your desired outcome.
- If your primary focus is preventing surface oxidation on metals like steel or copper: An inert atmosphere is essential for annealing or brazing without creating a destructive oxide layer.
- If your primary focus is processing highly reactive metals like titanium or certain alloys: A high-purity inert gas environment is non-negotiable to prevent a catastrophic loss of material integrity.
- If your primary focus is consistency in advanced manufacturing (e.g., sintering metal powders): The controlled environment of an inert furnace eliminates atmospheric variables, ensuring repeatable and predictable results.
Ultimately, an inert atmosphere furnace provides the control necessary to transform materials with heat without compromising their fundamental properties.
Summary Table:
| Feature | Purpose | Key Benefit |
|---|---|---|
| Inert Gas Environment | Replaces oxygen with non-reactive gases (Argon/Nitrogen) | Prevents oxidation and chemical contamination |
| Sealed Chamber | Contains the inert atmosphere and workpiece | Ensures process integrity and repeatability |
| High-Temperature Heating | Applies precise thermal energy to materials | Enables processes like sintering and annealing without material degradation |
Need precise, contamination-free heat treatment?
KINTEK's high-temperature inert atmosphere furnaces are engineered for demanding laboratory and production environments. Whether your work involves sensitive metal alloys, advanced ceramics, or R&D projects requiring absolute process control, our solutions deliver the reliable, repeatable results you need.
We specialize in providing lab equipment that empowers your research and manufacturing.
Contact our experts today to discuss how an inert atmosphere furnace can solve your specific material processing challenges.
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
People Also Ask
- What are the advantages of inert gas? Achieve Process Purity, Safety, and Material Protection
- Why use a tube atmosphere furnace with inert gas for catalyst calcination? Protect Active Sites from Contamination
- What is the role of an Atmosphere Furnace in the preparation of lignin-based graphene oxide? Key Carbonization Insights
- Why is nitrogen used in furnaces? Key Benefits for High-Temperature Processes
- Why do heat treat furnaces for specialty alloys often have atmosphere controls? To Protect and Transform Your Alloys
- What is a controlled atmosphere lab furnace? Master Material Protection and Transformation
- What role does an atmosphere sintering furnace play in NMC622 & LLZ co-sintering? Achieve High-Performance Interfaces
- Why is a high-temperature furnace with inert gas protection required for thermal aging of stainless steel?



















