In Chemical Vapor Deposition (CVD), a precursor is the starting chemical compound that contains the element or elements you wish to deposit as a thin film. This compound is introduced into a reactor in a gaseous or vapor state, where it undergoes a chemical reaction or decomposition on a heated substrate surface, leaving behind the desired solid material.
The precursor is not just an ingredient; it is the fundamental delivery vehicle for the atoms that will build your thin film. The choice of precursor dictates the process conditions, the quality of the final material, and the overall safety and cost of the operation.

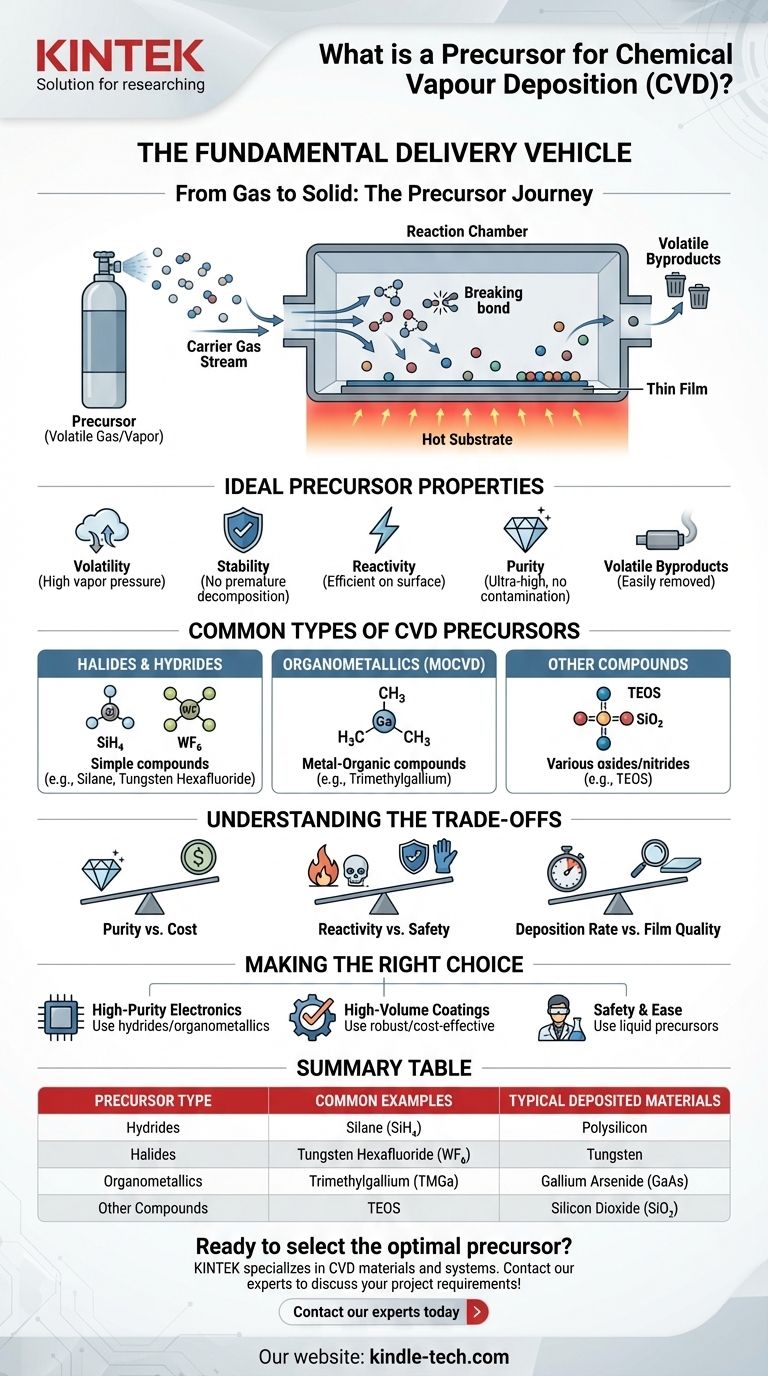
The Fundamental Role of a Precursor
To understand CVD, you must first understand the journey of the precursor. It is the core component that enables the transformation from a gas-phase chemical to a solid-phase material.
From Gas to Solid
The primary function of a precursor is to be volatile. It must be easily converted into a gas or vapor so it can be transported by a carrier gas to the substrate inside the reaction chamber.
Once at the hot substrate, the precursor's chemical bonds break. This decomposition process releases the desired atoms, which then deposit onto the surface, gradually building the thin film layer by layer.
What Makes a Good Precursor?
Not all chemicals are suitable precursors. An ideal precursor has a specific set of properties:
- Volatility: It must have a high enough vapor pressure to be transported as a gas at reasonable temperatures.
- Stability: It must be stable enough to travel to the substrate without decomposing prematurely in the gas stream.
- Reactivity: It must decompose cleanly and efficiently at the desired deposition temperature on the substrate surface.
- Purity: It must be available in a very high purity form to avoid contaminating the final film.
- Byproducts: It should produce volatile byproducts that can be easily removed from the chamber and do not interfere with the film growth.
Common Types of CVD Precursors
Precursors are generally categorized by their chemical nature. The choice depends entirely on the material you intend to deposit.
Halides and Hydrides
These are some of the most common and fundamental precursors. They are simple compounds formed between the element of interest and a halogen (like chlorine or fluorine) or hydrogen.
For example, polysilicon, a critical material for solar cells and microelectronics, is often deposited using silane (SiH₄), a hydride precursor. Halides like tungsten hexafluoride (WF₆) are used to deposit tungsten films.
Organometallics
Used in a sub-field of CVD called Metal-Organic CVD (MOCVD), these precursors are complex molecules containing a metal atom bonded to organic groups.
Organometallics are essential for depositing high-quality compound semiconductors used in LEDs and lasers. An example is using trimethylgallium (Ga(CH₃)₃) to provide the gallium for gallium arsenide (GaAs) films.
Other Compounds
A wide range of other chemicals serve as precursors for depositing oxides and nitrides. For instance, silicon dioxide (SiO₂), a common insulator in electronics, is typically deposited using tetraethyl orthosilicate (TEOS). TEOS is a liquid precursor that is less hazardous than silane, making it a popular choice.
Understanding the Trade-offs
Selecting a precursor is a balancing act between performance, cost, and safety. There is no single "best" precursor, only the most appropriate one for a specific application.
Purity vs. Cost
Ultra-high purity precursors are necessary for high-performance electronic devices but are significantly more expensive. For less critical applications like protective coatings, a lower-purity, lower-cost precursor may be sufficient.
Reactivity vs. Safety
Highly reactive precursors like hydrides (e.g., silane, arsine) enable efficient, low-temperature deposition. However, many are extremely toxic, flammable, or pyrophoric (ignite spontaneously in air), requiring expensive and complex safety and handling systems.
Deposition Rate vs. Film Quality
The type of precursor directly influences the deposition mechanism. As noted in Low-Pressure CVD (LPCVD), the process is often reaction rate limited, meaning the speed of the precursor's chemical reaction on the surface controls the growth. This slow, controlled process often yields high-quality, uniform films.
In contrast, some processes are mass transfer limited, where the rate is limited only by how fast the precursor can be supplied to the surface. This can lead to very fast deposition but may result in lower film quality and poor uniformity.
Making the Right Choice for Your Process
Your final goal for the thin film dictates the optimal precursor strategy.
- If your primary focus is high-purity, crystalline films for electronics: Choose well-characterized, high-purity precursors like specific hydrides or organometallics, even if they are more expensive or hazardous.
- If your primary focus is high-volume, functional coatings: Opt for more common, robust, and cost-effective precursors where minor impurities will not compromise the film's function.
- If your primary focus is process safety and ease of handling: Select less hazardous liquid precursors over highly toxic gases, accepting potential trade-offs in deposition temperature or film purity.
Ultimately, the precursor is the foundational choice that defines the potential and limitations of your entire CVD process.
Summary Table:
| Precursor Type | Common Examples | Typical Deposited Materials |
|---|---|---|
| Hydrides | Silane (SiH₄) | Polysilicon |
| Halides | Tungsten Hexafluoride (WF₆) | Tungsten |
| Organometallics | Trimethylgallium (TMGa) | Gallium Arsenide (GaAs) |
| Other Compounds | TEOS | Silicon Dioxide (SiO₂) |
Ready to select the optimal precursor for your CVD process? The right choice is critical for achieving high-purity, uniform thin films for electronics, optics, or protective coatings. KINTEK specializes in lab equipment and consumables, serving laboratory needs with expert guidance on CVD materials and systems. Let our specialists help you balance performance, safety, and cost for your specific application. Contact our experts today to discuss your project requirements!
Visual Guide
Related Products
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
- CVD Diamond Domes for Industrial and Scientific Applications
- Glassy Carbon Sheet RVC for Electrochemical Experiments
- Vacuum Cold Trap Direct Cold Trap Chiller
People Also Ask
- What are the sputtering parameters for thin films? Master the Keys to Precise Film Deposition
- What is a sputtering target used for? The Atomic Blueprint for High-Performance Thin Films
- What are the applications of carbon nanomaterials? Unlock Revolutionary Performance in Energy, Materials & Electronics
- What is the difference between sputtering and electron beam evaporation? Choose the Right PVD Method
- What is the synthesis process of graphene? A Guide to Top-Down and Bottom-Up Methods
- What are the advantages of carbon nanotubes? Unlock Superior Strength, Conductivity & Performance
- What is meant by thin film in optics? Control Light with Nanoscale Precision
- What are the properties of diamond coating? Unlock Extreme Performance for Your Components



















