Fundamentally, an electrolysis cell is a device that uses electrical energy to drive a chemical reaction that would not otherwise occur on its own. It effectively reverses the process of a battery, consuming power to decompose compounds or synthesize new ones. This is achieved by passing a direct electrical current through a substance called an electrolyte, forcing chemical changes at two electrodes.
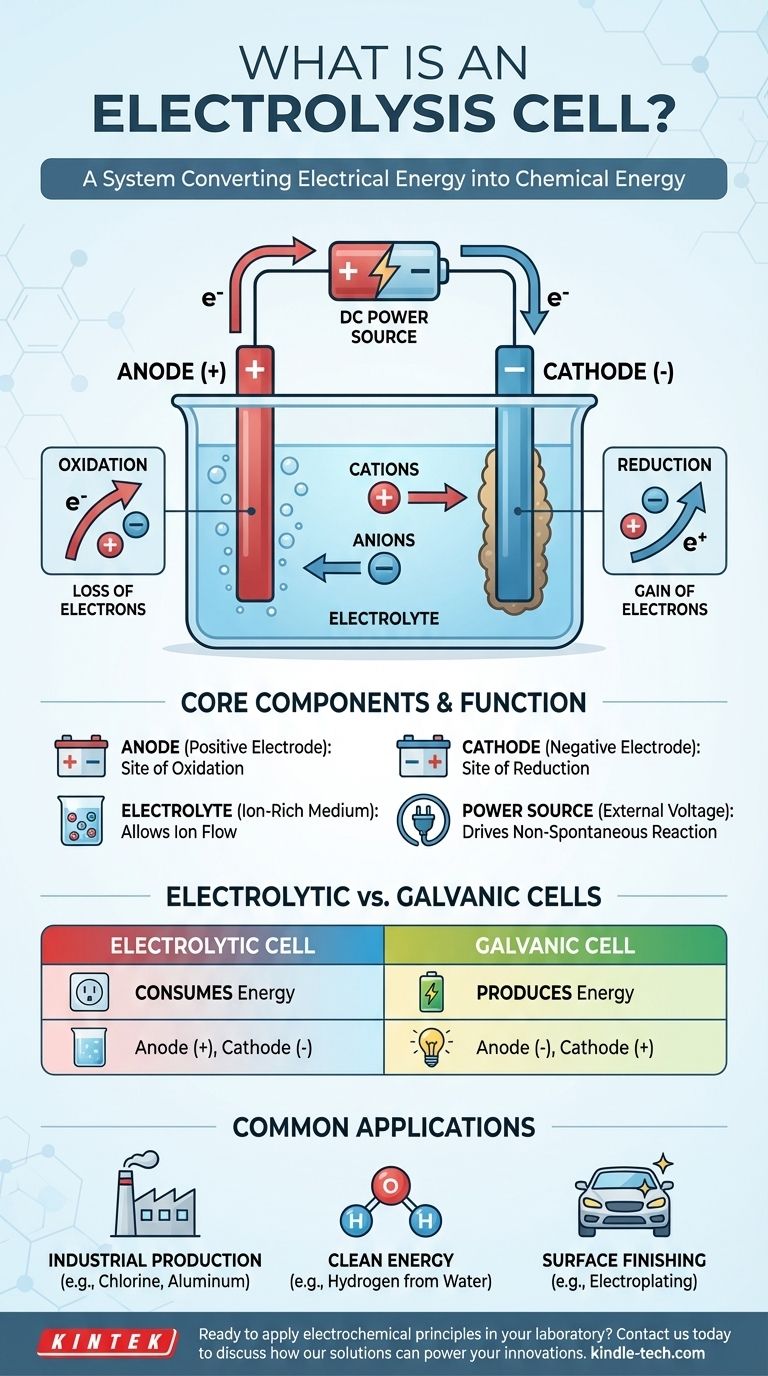
An electrolysis cell is best understood as a system for converting electrical energy into chemical energy. It functions by applying an external voltage to overcome a chemical system's natural state, forcing ions to react and create new substances.
How an Electrolysis Cell Works
To grasp the concept, it's essential to understand the three core components and how they interact when electricity is introduced. The process is a controlled and directed flow of charge that results in chemical transformation.
The Three Essential Components
An electrolytic cell is comprised of three primary parts:
- An Anode: The electrode that is connected to the positive terminal of the power source.
- A Cathode: The electrode that is connected to the negative terminal of the power source.
- An Electrolyte: A substance containing free-moving ions, such as a molten salt or an ionic compound dissolved in a solvent like water.
The Role of External Voltage
Unlike a battery (a galvanic cell) which produces voltage spontaneously, an electrolytic cell requires an external power source. This applied voltage establishes a powerful electric field and dictates the polarity of the electrodes, making the anode positive and the cathode negative.
The Flow of Ions and Electrons
The applied voltage forces ions within the electrolyte to migrate. Positively charged ions (cations) are drawn toward the negative cathode, while negatively charged ions (anions) are drawn toward the positive anode. This migration sets the stage for the chemical reactions.
Oxidation and Reduction: The Core Chemistry
The actual chemical change happens at the surface of the electrodes. These reactions are known as "redox" reactions, short for reduction and oxidation.
The Anode: Site of Oxidation
The anode is defined as the site of oxidation. Here, the anions that have migrated to the positive electrode give up their excess electrons. This loss of electrons is the definition of oxidation.
The Cathode: Site of Reduction
The cathode is defined as the site of reduction. Cations drawn to this negative electrode accept electrons from the circuit. This gain of electrons is the definition of reduction, often resulting in the deposition of a neutral element (like a metal) onto the cathode.
Understanding the Key Distinction: Electrolytic vs. Galvanic Cells
A common point of confusion is the difference between an electrolytic cell and a galvanic cell (like a common battery). Understanding their opposing functions is critical.
Energy Consumption vs. Energy Production
The most important difference is energy flow. An electrolytic cell consumes energy to drive a non-spontaneous reaction. A galvanic cell releases energy from a spontaneous reaction.
Electrode Polarity
This difference in function leads to an opposite sign convention for the electrodes.
- In an electrolytic cell, the anode is positive and the cathode is negative.
- In a galvanic cell, the anode is negative and the cathode is positive.
Remember that in both cell types, oxidation always occurs at the anode and reduction always occurs at the cathode. The polarity is what changes.
Common Applications of Electrolysis
The ability to force chemical change with electricity makes electrolytic cells incredibly useful across many industries.
Industrial Production
Electrolysis is the cornerstone of the chlor-alkali industry, which produces chlorine gas and sodium hydroxide. It is also essential for producing pure metals like aluminum and copper from their ores.
Clean Energy
One of the most promising applications is the electrolysis of water. By passing a current through water, it can be split into its constituent elements: hydrogen and oxygen. The resulting hydrogen can be used as a clean fuel.
Surface Finishing
Electroplating is a common process where an electrolytic cell is used to deposit a thin layer of one metal (like gold or chromium) onto the surface of another object, which acts as the cathode.
Making the Right Choice for Your Goal
Understanding the principle of electrolysis allows you to apply it to specific objectives.
- If your primary focus is material production (e.g., hydrogen): Recognize that the cell is a tool to break down stable, low-energy compounds (like water) into high-energy, valuable products (like H₂ gas).
- If your primary focus is surface coating (electroplating): Understand that your target object must be the cathode, as this is where positive metal ions will be reduced and deposited as a solid layer.
- If your primary focus is fundamental electrochemistry: The most crucial takeaway is that an electrolytic cell uses external energy to reverse a natural chemical process, making it a powerful engine for synthesis.
By mastering these principles, you can leverage electrolysis as a powerful and precise tool for chemical transformation.
Summary Table:
| Component | Function | Key Characteristic |
|---|---|---|
| Anode | Site of Oxidation (loss of electrons) | Connected to the positive terminal of the power source |
| Cathode | Site of Reduction (gain of electrons) | Connected to the negative terminal of the power source |
| Electrolyte | Provides medium for ion flow | A molten salt or ionic solution containing free-moving ions |
| Power Source | Drives the non-spontaneous reaction | Provides the external voltage required for electrolysis |
Ready to apply electrochemical principles in your laboratory?
Whether your project involves material synthesis, electroplating, or developing clean energy solutions, having the right equipment is fundamental. KINTEK specializes in providing high-quality lab equipment and consumables to support your electrochemical research and development.
Contact us today to discuss how our solutions can power your innovations and provide the precision and reliability your laboratory needs.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell with Five-Port
- H Type Electrolytic Cell Triple Electrochemical Cell
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
- Electrolytic Electrochemical Cell for Coating Evaluation
People Also Ask
- How should the five-port water bath electrolytic cell be cleaned for maintenance? A Step-by-Step Guide to Reliable Results
- How can leaks be prevented when using a five-port water bath electrolytic cell? Ensure a Reliable and Safe Electrochemical Setup
- What is the proper way to handle a five-port water bath electrolytic cell? Ensure Accurate and Safe Electrochemical Experiments
- How can contamination be avoided during experiments with the five-port water bath electrolytic cell? Master the 3-Pillar Protocol
- What are the standard components of the five-port water bath electrolytic cell? Master the Precision Instrument for Electrochemical Analysis



















