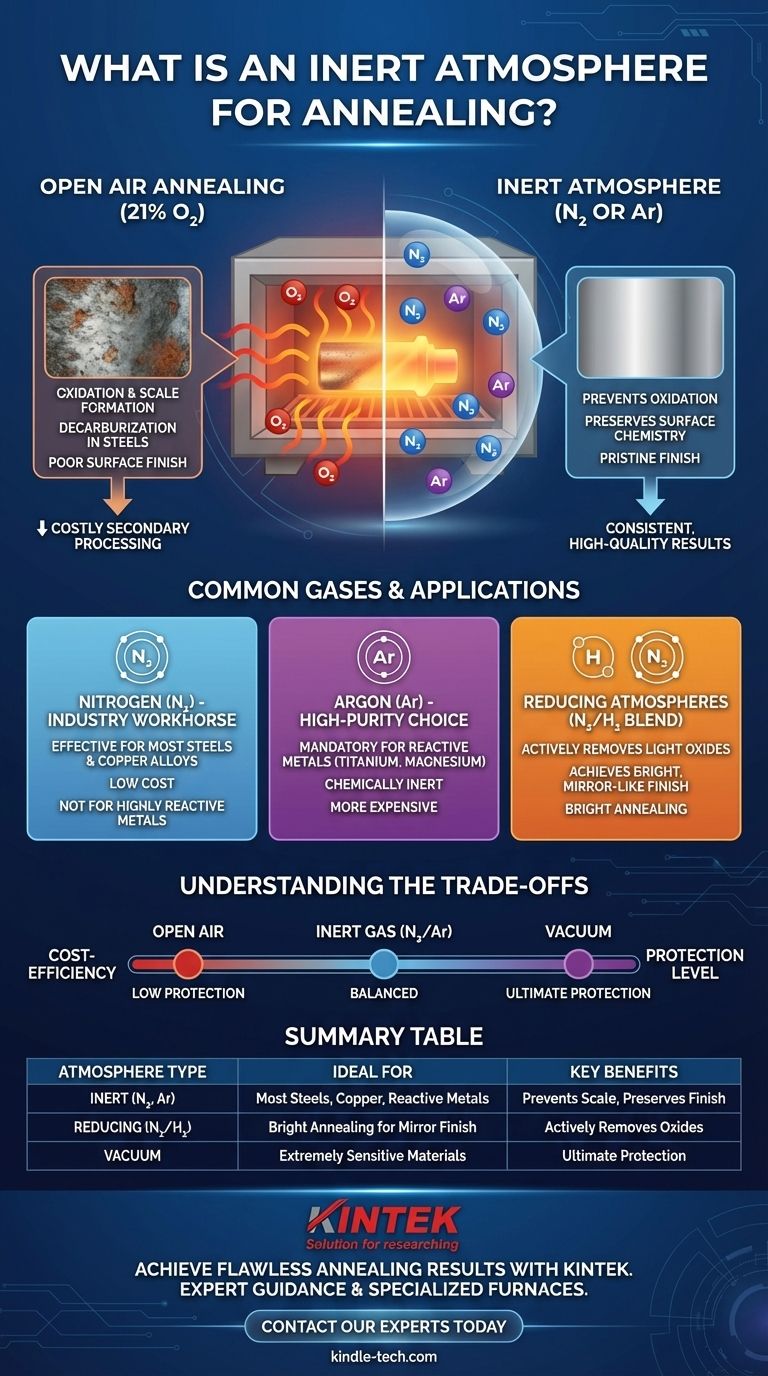
In short, an inert atmosphere for annealing is a carefully controlled gaseous environment inside a furnace that will not chemically react with the material being heat-treated. This protective blanket of gas, typically Nitrogen or Argon, prevents destructive surface reactions like oxidation (rusting or scaling) that would otherwise occur at the high temperatures required for annealing.
The core purpose of using an inert atmosphere is to preserve the material's intended surface finish and chemical composition. While annealing softens the metal and refines its internal structure, the inert gas acts as a shield, ensuring the part exits the furnace in the same pristine surface condition it entered.

Why an Inert Atmosphere is Critical
To understand the need for an inert atmosphere, you must first recognize that heat acts as a powerful catalyst for chemical reactions. A metal that is stable at room temperature becomes highly reactive when heated.
The Problem: High Temperatures and Reactivity
Annealing requires heating metals to hundreds or even thousands of degrees. At these temperatures, metal atoms are highly energized and eager to react with any available elements in their environment.
The most common and problematic element is the oxygen present in normal air (which is roughly 21% oxygen).
Preventing Oxidation and Scale Formation
When a hot metal surface is exposed to oxygen, a chemical reaction called oxidation occurs almost instantly. This forms a hard, brittle layer of metallic oxides on the surface, commonly known as scale.
This scale layer is almost always undesirable. It ruins the surface finish, must be removed through costly secondary processes like sandblasting or acid pickling, and represents a loss of base material. Using an inert atmosphere displaces the oxygen, preventing scale from ever forming.
Avoiding Decarburization in Steels
For carbon steels, there is an additional risk: decarburization. At high temperatures, the carbon within the steel can react with oxygen, pulling the carbon out of the surface and leaving it as carbon monoxide (CO) or carbon dioxide (CO₂) gas.
This loss of carbon softens the surface layer of the steel, which can be detrimental to the final part's wear resistance and fatigue life. An inert atmosphere protects the surface chemistry, ensuring the carbon stays where it belongs.
Common Gases Used for Inert Atmospheres
The choice of gas depends on the material being treated, the required purity, and the cost.
Nitrogen (N₂): The Industry Workhorse
Nitrogen is the most widely used gas for creating a protective atmosphere. It is effective for annealing most common materials, including copper, bronze, and the majority of carbon and alloy steels.
Its primary advantage is its low cost, as it can be separated directly from the air. However, for certain highly reactive materials, nitrogen is not truly inert and can form unwanted nitrides.
Argon (Ar): The High-Purity Choice
Argon is a noble gas, meaning it is chemically inert under almost all conditions. It is the mandatory choice for annealing reactive metals like titanium, magnesium, and certain high-alloy or stainless steels.
While it provides superior protection, Argon is significantly more expensive than nitrogen because it is less abundant in the atmosphere.
Beyond Inert: Reducing Atmospheres
Sometimes, a small amount of a reactive gas like hydrogen (H₂) is intentionally added to the nitrogen or argon base. This creates a reducing atmosphere.
Instead of just preventing oxidation, a reducing atmosphere will actively strip any trace oxygen from the furnace and can even reduce light oxides already present on the part's surface. This process, often called bright annealing, results in an exceptionally clean and bright surface finish.
Understanding the Trade-offs
Choosing a furnace atmosphere is a balance between metallurgical requirements, surface finish quality, and operational cost.
Inert Atmosphere vs. Open Air
Annealing in open air is the cheapest possible method but offers zero protection. It is only suitable for parts where heavy surface scale is acceptable or for materials that will be fully machined after heat treatment, removing the damaged surface layer entirely.
Inert Atmosphere vs. Vacuum
Vacuum annealing provides the highest level of protection by removing virtually all gas molecules from the furnace chamber. It is the ultimate solution for extremely sensitive materials.
However, vacuum furnaces are more expensive to build and operate, and their process cycles are typically slower than annealing in an inert gas at positive pressure.
The Purity Factor: Why "Parts Per Million" Matters
Even within an "inert" atmosphere, trace amounts of oxygen or moisture can cause discoloration or light oxidation. The purity of the supplied gas and the integrity of the furnace are critical. For high-value components, specifying gas purity in parts per million (PPM) of contaminants is standard practice.
Choosing the Right Atmosphere for Your Process
Your choice of atmosphere directly impacts the quality of your final product and your operational budget.
- If your primary focus is cost-efficiency for common steels or copper alloys: A pure Nitrogen atmosphere is the most effective and economical choice.
- If you are working with reactive metals like titanium or specific stainless steel grades: You must use Argon to prevent unwanted nitride formation.
- If your goal is the cleanest possible, mirror-like surface finish: A reducing atmosphere (like a Nitrogen-Hydrogen blend) or vacuum annealing is necessary.
- If the part will be fully machined after annealing: You may be able to anneal in open air, but you must account for material loss and the cost of scale removal.
Controlling the furnace atmosphere is not an afterthought; it is a fundamental tool for achieving precise metallurgical outcomes.
Summary Table:
| Atmosphere Type | Common Gases | Key Benefits | Ideal For |
|---|---|---|---|
| Inert | Nitrogen (N₂), Argon (Ar) | Prevents oxidation/scale, preserves surface finish | Most steels, copper alloys (N₂); Reactive metals like titanium (Ar) |
| Reducing | N₂/H₂ or Ar/H₂ blend | Actively removes light oxides, achieves bright finish | Bright annealing for a mirror-like surface |
| Vacuum | N/A (High Vacuum) | Ultimate protection for sensitive materials | Extremely reactive or high-purity applications |
| Open Air | Air (21% O₂) | Lowest cost | Parts that will be fully machined post-annealing |
Achieve Flawless Annealing Results with KINTEK
Selecting the right atmosphere is critical to your heat treatment success. Whether you're annealing common steels, reactive alloys, or aiming for a bright finish, KINTEK has the expertise and equipment to meet your lab's specific needs.
We provide:
- Specialized Annealing Furnaces designed for precise atmosphere control.
- Expert Guidance to help you choose between Nitrogen, Argon, or reducing atmospheres for optimal results and cost-efficiency.
- Reliable Consumables to ensure your process runs smoothly.
Let's protect your materials and perfect your process. Contact our annealing experts today to discuss your application!
Visual Guide
Related Products
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- 1400℃ Laboratory High Temperature Tube Furnace with Alumina Tube
People Also Ask
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- How do you make an inert atmosphere? Master Safe, Pure Processes with Inerting
- How we can develop inert atmosphere for a chemical reaction? Master Precise Atmospheric Control for Your Lab
- How does an atmosphere furnace facilitate the post-treatment of nickel-plated carbon fibers? Ensure Peak Bonding
- What is the role of an atmosphere-controlled tube furnace in Cu-Mo sintering? Achieve High-Purity Densification



















