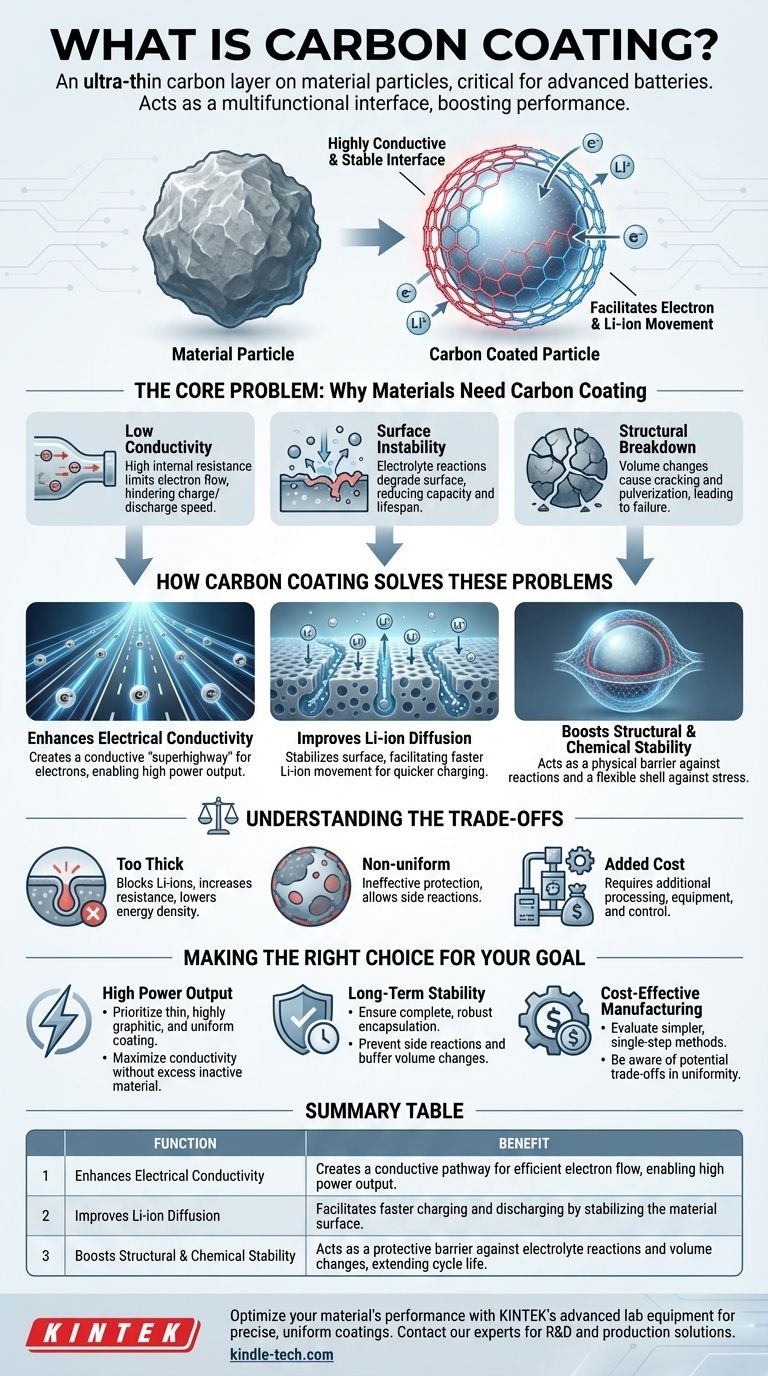
In essence, carbon coating is the process of applying an ultra-thin layer of carbon onto the surface of another material's particles. This technique is especially critical for advanced battery electrode materials, where it acts as a multifunctional interface to solve inherent limitations and dramatically boost performance.
While often viewed as a simple protective layer, carbon coating is a sophisticated engineering solution. Its true purpose is to fundamentally improve a material's electrochemical performance by creating a stable, highly conductive surface that facilitates both electron and ion movement.
The Core Problem: Why Materials Need Carbon Coating
Many materials with high potential for energy storage, particularly in lithium-ion batteries, suffer from critical flaws that prevent their practical use. Carbon coating is a targeted solution to these exact problems.
The Challenge of Low Conductivity
Many promising electrode materials, such as Lithium Iron Phosphate (LFP) or silicon, are inherently poor electrical conductors. This high internal resistance acts as a bottleneck, limiting the flow of electrons and severely hindering the battery's ability to charge and discharge quickly.
The Instability of Electrode Surfaces
Electrode materials are in constant contact with a reactive liquid electrolyte. This can trigger unwanted chemical side reactions that consume lithium and form a resistive layer on the particle surface, degrading battery capacity and lifespan over time.
The Breakdown of Structural Integrity
Some materials, like silicon, undergo massive volume expansion and contraction during charging and discharging. This repeated stress can cause the particles to crack and pulverize, leading to a rapid loss of electrical contact and catastrophic failure of the cell.
How Carbon Coating Solves These Problems
A well-designed carbon coating directly addresses the issues of conductivity and stability. It functions as a highly engineered interface between the active material and its surrounding environment.
Enhancing Electrical Conductivity
The carbon layer itself is highly conductive. It forms a continuous electronic pathway around the otherwise resistive particle, creating a "superhighway" for electrons to travel to and from the material with minimal resistance. This is essential for achieving high power output.
Improving Li-ion Diffusion
The coating doesn't just help electrons; it helps lithium ions. By creating a stable and well-structured surface, it facilitates the efficient movement of Li-ions into and out of the host material. This directly improves the rate at which the battery can be charged and discharged.
Boosting Structural and Chemical Stability
The carbon coating acts as a physical barrier. It shields the active material from direct contact with the electrolyte, which modifies its surface chemical stability and suppresses performance-degrading side reactions. For materials that expand, the coating also acts as a mechanically flexible shell, helping to enhance structural stability and hold the particle together.
Understanding the Trade-offs
Applying a carbon coating is not a magic bullet. The quality and characteristics of the coating are critical, and a poor implementation can create more problems than it solves.
The Risk of an Excessively Thick Coating
A coating that is too thick can begin to block the pathways for lithium ions, paradoxically increasing resistance and slowing down performance. Furthermore, carbon is an "inactive" material—it doesn't store lithium—so excess carbon lowers the overall energy density of the battery.
The Importance of Coating Uniformity
A patchy or non-uniform coating is ineffective. Uncoated areas remain exposed to the electrolyte, allowing side reactions to occur and defeating the coating's protective purpose. Achieving a perfectly uniform layer is a significant manufacturing challenge.
The Added Manufacturing Cost
Applying a consistent, high-quality carbon coating requires additional processing steps, sophisticated equipment, and precise control. This inevitably adds complexity and cost to the final material production.
Making the Right Choice for Your Goal
The ideal carbon coating strategy depends entirely on the specific performance characteristic you are trying to optimize.
- If your primary focus is high power output: Prioritize a thin, highly graphitic, and uniform coating to maximize both electronic conductivity and Li-ion diffusion without adding excess inactive material.
- If your primary focus is long-term stability and cycle life: Ensure the coating provides complete, robust encapsulation to prevent side reactions and buffer against any volume changes during operation.
- If your primary focus is cost-effective manufacturing: Evaluate simpler, single-step coating methods, but remain aware of the potential trade-off in coating uniformity and the resulting electrochemical performance.
Ultimately, a well-designed carbon coating transforms a promising material into a high-performance component, bridging the gap between intrinsic properties and practical application.
Summary Table:
| Function | Benefit |
|---|---|
| Enhances Electrical Conductivity | Creates a conductive pathway for efficient electron flow, enabling high power output. |
| Improves Li-ion Diffusion | Facilitates faster charging and discharging by stabilizing the material surface. |
| Boosts Structural & Chemical Stability | Acts as a protective barrier against electrolyte reactions and volume changes, extending cycle life. |
Ready to optimize your material's performance with a tailored carbon coating solution? KINTEK specializes in advanced lab equipment and consumables for precise material processing. Whether you're developing next-generation battery electrodes or enhancing material properties, our expertise ensures you achieve uniform, high-quality coatings for superior conductivity and stability. Contact our experts today to discuss how we can support your R&D and production goals.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Cylindrical Resonator MPCVD Machine System Reactor for Microwave Plasma Chemical Vapor Deposition and Lab Diamond Growth
People Also Ask
- How does PECVD work? Enable Low-Temperature, High-Quality Thin Film Deposition
- What are the methods of deposition? A Guide to PVD and CVD Thin-Film Techniques
- What color diamonds are CVD? Understanding the Process from Brown Tint to Colorless Beauty
- What are the different types of thin films? A Guide to Optical, Electrical, and Functional Coatings
- What are the steps of the CVD process? A Guide to Precision Thin Film Deposition



















