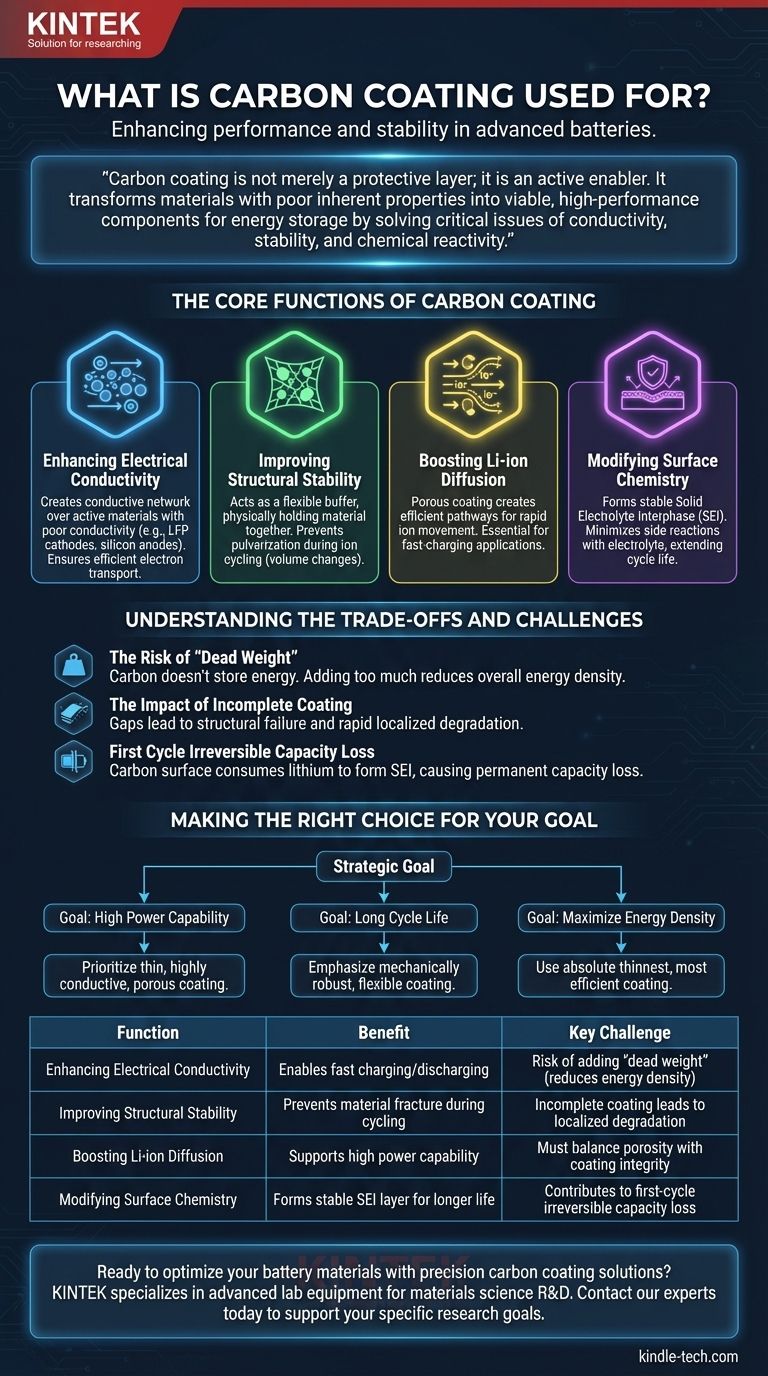
In materials science, carbon coating is primarily used to enhance the performance and stability of electrode materials, especially within advanced batteries like lithium-ion systems. It addresses fundamental limitations by modifying the material's surface to improve electrical conductivity, provide structural support during ion cycling, and create a more stable interface with the electrolyte.
Carbon coating is not merely a protective layer; it is an active enabler. It transforms materials with poor inherent properties into viable, high-performance components for energy storage by solving critical issues of conductivity, stability, and chemical reactivity.

The Core Functions of Carbon Coating
To understand why carbon coating is so critical, we must look at the fundamental problems it solves in active materials, particularly for battery anodes and cathodes.
Enhancing Electrical Conductivity
Many promising electrode materials, such as Lithium Iron Phosphate (LFP) cathodes or silicon anodes, have poor intrinsic electrical conductivity. This limits how quickly they can be charged and discharged.
Carbon, in forms like amorphous carbon or graphite, is an excellent electrical conductor. By applying a thin, uniform carbon layer, a conductive network is created across the surface of the active material particles, ensuring efficient electron transport to and from the current collector.
Improving Structural Stability
Advanced materials often experience significant volume changes during the insertion and extraction of ions (e.g., lithium ions). This expansion and contraction can cause the material to fracture and lose electrical contact over time.
A well-engineered carbon coating acts as a mechanically flexible buffer. It physically holds the active material together, preventing pulverization and preserving the electrode's integrity through thousands of charge-discharge cycles.
Boosting Li-ion Diffusion
The speed at which ions can move into and out of the active material is a key factor in a battery's power capability. A dense or poorly structured surface can impede this movement.
A porous carbon coating can be designed to create efficient pathways for Li-ion diffusion. This ensures that ions can quickly reach the active material, which is essential for fast-charging applications.
Modifying Surface Chemistry
The surface of an electrode is a highly reactive environment. Unwanted side reactions with the liquid electrolyte can consume active lithium and degrade the battery's capacity and safety over time.
Carbon coating helps form a more stable and uniform Solid Electrolyte Interphase (SEI) layer. This controlled interface minimizes parasitic reactions, leading to significantly longer cycle life and improved overall stability.
Understanding the Trade-offs and Challenges
While beneficial, carbon coating is not a magic bullet. Its application requires careful engineering to balance its advantages against its inherent drawbacks.
The Risk of "Dead Weight"
The carbon coating itself does not store energy. Adding too much carbon increases the total weight and volume of the electrode without contributing to its capacity.
This directly reduces the cell's overall energy density (the amount of energy stored per unit of weight or volume). The goal is to use the absolute minimum amount of carbon required to achieve the desired performance benefits.
The Impact of Incomplete Coating
The benefits of carbon coating are only realized if the layer is uniform and complete. Any gaps or bare spots on the active material become weak points.
These uncoated areas are susceptible to structural failure and aggressive side reactions with the electrolyte, undermining the purpose of the coating and leading to rapid localized degradation.
First Cycle Irreversible Capacity Loss
When a battery is first charged, a portion of the lithium ions is consumed to form the SEI layer on the surface of the anode. The carbon coating has a high surface area and also participates in this reaction.
This process leads to a permanent loss of some of the battery's charge capacity, known as first-cycle irreversible capacity loss. Optimizing the coating is crucial to minimizing this effect.
Making the Right Choice for Your Goal
The ideal carbon coating strategy depends entirely on the primary objective for the material you are developing.
- If your primary focus is high power capability: Prioritize a thin, highly conductive carbon layer that maximizes electron transport and provides porous channels for rapid ion diffusion.
- If your primary focus is long cycle life: Emphasize a mechanically robust and flexible coating that can withstand significant volume expansion and prevent material pulverization.
- If your primary focus is maximizing energy density: Use the absolute thinnest, most efficient coating possible to minimize "dead weight" and reduce first-cycle irreversible capacity loss.
Ultimately, carbon coating is a strategic tool that allows material scientists and engineers to unlock the potential of advanced materials for next-generation energy storage.
Summary Table:
| Function | Benefit | Key Challenge |
|---|---|---|
| Enhancing Electrical Conductivity | Enables fast charging/discharging | Risk of adding 'dead weight' (reduces energy density) |
| Improving Structural Stability | Prevents material fracture during cycling | Incomplete coating leads to localized degradation |
| Boosting Li-ion Diffusion | Supports high power capability | Must balance porosity with coating integrity |
| Modifying Surface Chemistry | Forms stable SEI layer for longer life | Contributes to first-cycle irreversible capacity loss |
Ready to optimize your battery materials with precision carbon coating solutions? KINTEK specializes in advanced lab equipment and consumables for materials science R&D. Whether you're developing next-generation anodes, cathodes, or other energy storage components, our tools help you achieve uniform, high-performance coatings that balance conductivity, stability, and energy density. Contact our experts today to discuss how we can support your specific research goals and accelerate your path to innovation.
Visual Guide
Related Products
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- CVD Diamond Cutting Tool Blanks for Precision Machining
- Anti-Cracking Press Mold for Lab Use
- Laboratory Sterilizer Lab Autoclave Pulse Vacuum Lifting Sterilizer
People Also Ask
- How does PACVD equipment improve DLC coatings? Unlock Low Friction and High Heat Resistance
- What are the advantages of using HFCVD for BDD electrodes? Scaling Industrial Diamond Production Efficiently
- How is something diamond coated? A Guide to CVD Growth vs. Plating Methods
- What is microwave plasma CVD? A Guide to High-Purity Diamond and Material Synthesis
- What is the specific function of the metal filament in HF-CVD? Key Roles in Diamond Growth



















