In short, Chemical Vapor Deposition (CVD) is a highly versatile manufacturing process used to create exceptionally thin, high-performance coatings on a wide range of materials. It is the foundational technique for building modern electronics by depositing films on semiconductor wafers, but it is also used to create wear-resistant coatings for cutting tools and to produce the photovoltaic materials in thin-film solar cells.
The core value of CVD lies in its ability to build a coating from a gas, one molecule at a time. This allows it to create incredibly pure, durable, and perfectly uniform films that can conform to even the most complex surfaces, a feat many other coating methods cannot achieve.

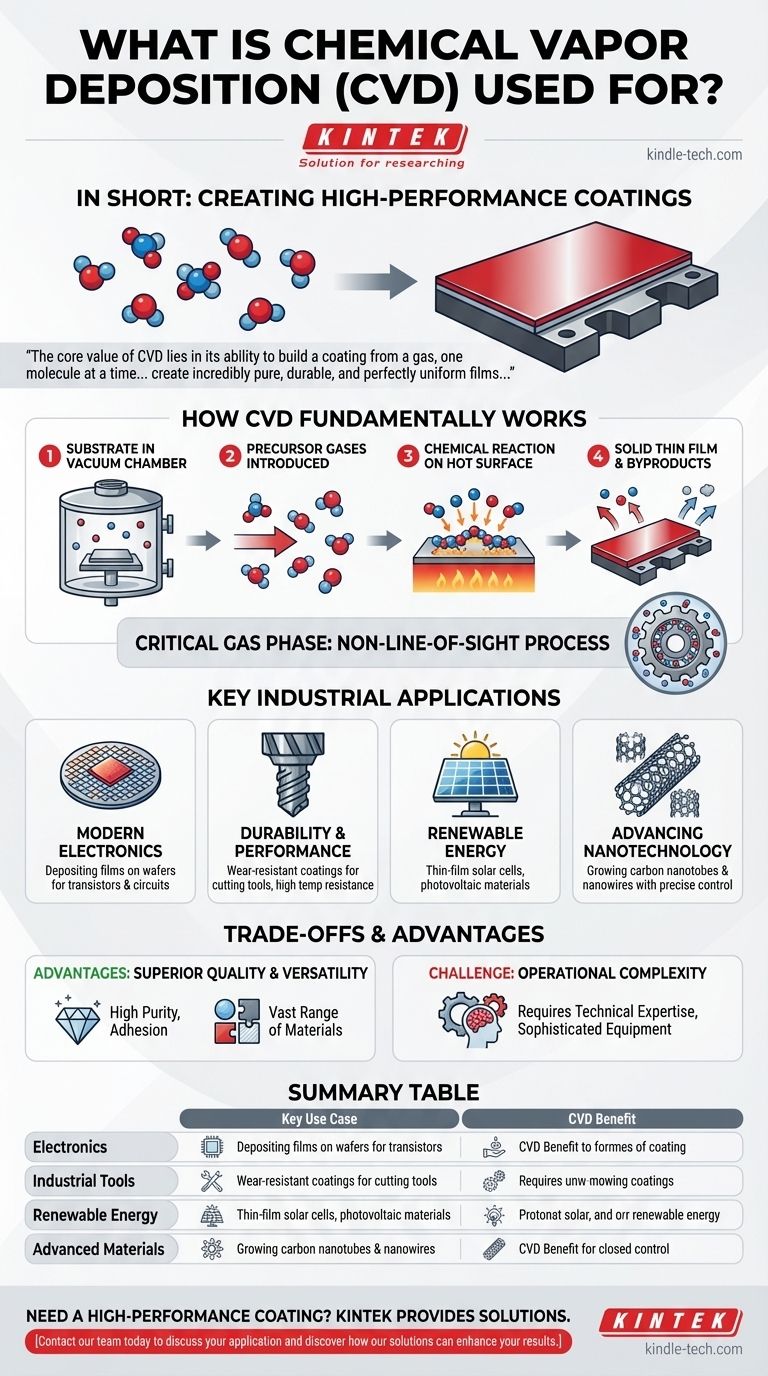
How Chemical Vapor Deposition Fundamentally Works
To understand its applications, you must first understand the process itself. CVD is not a simple spray-on or dipping method; it is a controlled chemical reaction on a surface.
The Core Process
A substrate, or the part to be coated, is placed inside a reaction chamber under a vacuum. Specific precursor gases containing the desired coating elements are then introduced into the chamber.
These gases react and decompose on the hot surface of the substrate, leaving behind a solid, high-purity thin film. The byproduct gases are then extracted from the chamber.
Why the Gas Phase is Critical
Because the coating material is delivered as a gas, it can penetrate and coat every exposed area of a complex part. This is known as a non-line-of-sight process, ensuring a completely uniform and conformal coating, even on intricate shapes.
Key Industrial Applications of CVD
The unique capabilities of CVD make it indispensable across several high-technology sectors where material performance at the microscopic level is critical.
The Foundation of Modern Electronics
Nearly every advanced microchip relies on CVD. The process is used to deposit various semiconducting, insulating, and metallic thin films onto silicon wafers, building the intricate layered structures that form transistors and circuits.
Enhancing Durability and Performance
For industrial applications, CVD is used to apply hard, durable coatings to cutting tools and mechanical parts. These coatings provide exceptional resistance to abrasion, corrosion, and high temperatures, dramatically extending the life and performance of the tool.
Powering Renewable Energy
CVD is essential in the manufacturing of thin-film solar cells. It is used to deposit the critical layers of photovoltaic material onto a substrate like glass, which are responsible for converting sunlight into electricity.
Advancing Nanotechnology
On the cutting edge of materials science, CVD is a primary method for growing advanced materials like carbon nanotubes and various nanowires. The process allows for the precise control needed to build these structures from the ground up.
Understanding the Trade-offs and Advantages
No single manufacturing process is perfect for every scenario. Understanding the specific benefits and inherent challenges of CVD is key to knowing when to apply it.
Advantage: Superior Coating Quality
CVD produces films of exceptionally high purity with excellent adhesion to the substrate. The coatings are dense and durable, capable of withstanding extreme temperatures and high-stress environments.
Advantage: Unmatched Versatility
The process can be used to deposit a vast range of materials, including metals, ceramics, and semiconductors. It also works on many different substrates, from metals and ceramics to glass.
Challenge: Operational Complexity
The primary trade-off is the skill required to run the process. CVD equipment is sophisticated, and optimizing the gas mixtures, temperatures, and pressures to achieve a perfect coating requires a high level of technical expertise.
Making the Right Choice for Your Goal
Selecting a coating technology depends entirely on your primary objective. CVD is the superior choice when surface performance and precision are non-negotiable.
- If your primary focus is extreme durability: CVD is ideal for creating wear- and corrosion-resistant coatings on tools and components in high-stress environments.
- If your primary focus is high purity and electrical performance: CVD is the industry standard for depositing the foundational layers of semiconductors and electronics.
- If your primary focus is uniform coverage on a complex shape: CVD's non-line-of-sight nature ensures a perfectly conformal coating that other methods cannot replicate.
Ultimately, Chemical Vapor Deposition is the enabling technology behind many of the advanced materials that define our modern world.
Summary Table:
| Application Area | Key Use Case | CVD Benefit |
|---|---|---|
| Electronics | Depositing films on semiconductor wafers | High purity, precise electrical properties |
| Industrial Tools | Wear-resistant coatings for cutting tools | Extreme durability, high-temperature resistance |
| Renewable Energy | Manufacturing thin-film solar cells | Uniform, conformal coatings on large areas |
| Advanced Materials | Growing carbon nanotubes and nanowires | Precise atomic-level control |
Need a high-performance coating for your project?
The unique advantages of Chemical Vapor Deposition—exceptional purity, perfect uniformity, and the ability to coat complex shapes—make it the ideal solution for demanding applications in semiconductors, industrial tooling, and renewable energy.
At KINTEK, we specialize in providing advanced lab equipment and consumables to meet your precise laboratory needs. Let our experts help you determine if CVD is the right technology for your specific material challenge.
Contact our team today to discuss your application and discover how our solutions can enhance your results.
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- HFCVD Machine System Equipment for Drawing Die Nano-Diamond Coating
- 915MHz MPCVD Diamond Machine Microwave Plasma Chemical Vapor Deposition System Reactor
- Vacuum Hot Press Furnace Machine for Lamination and Heating
- 1200℃ Split Tube Furnace with Quartz Tube Laboratory Tubular Furnace
People Also Ask
- What are the precursors used in CVD synthesis of graphene? Key Choices for High-Quality Growth
- What is the magnetron sputtering method of deposition? A Guide to High-Performance Thin Film Coating
- What are the steps of chemical vapour deposition? A Guide to the 7-Step CVD Process
- What is the difference between selective laser sintering and electron beam melting? Sintering vs. Melting for Additive Manufacturing
- What industries utilize the vacuum deposition process? Unlocking Precision in Electronics, Energy, and Healthcare
- Does the temperature increase or decrease in deposition? Understand the Thermodynamics of Phase Change
- What are the steps of sputtering? A Guide to Thin Film Deposition
- What is the process of deposition in semiconductors? Build Precise Thin Films for Your ICs



















