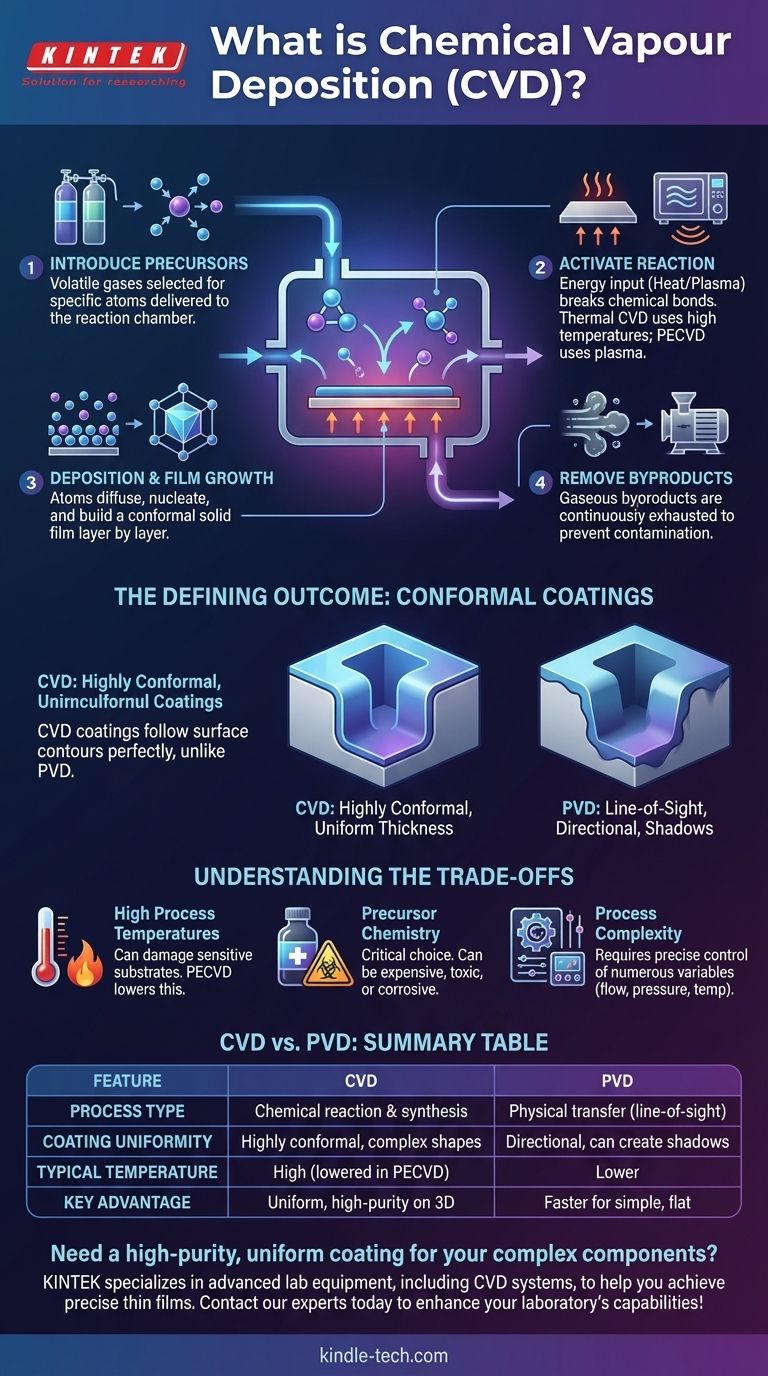
In essence, Chemical Vapor Deposition (CVD) is a process that builds a high-purity, solid thin film on a surface using a controlled chemical reaction. It begins by introducing volatile precursor gases into a reaction chamber containing the object to be coated, known as the substrate. Energy, typically in the form of heat, is applied to the substrate, causing the precursor gases to react or decompose on its surface, depositing a solid material and forming the desired film. Gaseous byproducts from the reaction are then exhausted from the chamber.
At its core, CVD is fundamentally different from physical coating methods. Instead of simply depositing an existing material, it synthesizes a new material directly onto a surface through a chemical transformation, allowing for exceptionally uniform and complex coatings.

The Core Principle: A Controlled Chemical Reaction
To truly understand CVD, you must think of it as orchestrating a chemical reaction where the target surface, or substrate, acts as the reaction site. Every step is designed to control this synthesis with high precision.
Step 1: Introducing the Precursors
The process begins with one or more volatile precursor gases. These are chemical compounds, often in liquid or solid form, that are vaporized and then precisely delivered into the reaction chamber. These gases are selected because they contain the specific atoms required for the final film.
Step 2: Activating the Reaction
For the precursors to react, they need an input of energy. In traditional thermal CVD, the substrate is heated to very high temperatures. This thermal energy breaks the chemical bonds in the precursor molecules when they come into contact with the hot surface.
Alternatively, methods like Plasma-Enhanced CVD (PECVD) use microwave or radio-frequency energy to generate a plasma—an ionized gas. This plasma creates highly reactive chemical species without requiring extremely high substrate temperatures.
Step 3: Deposition and Film Growth
Once the precursor gases decompose on the substrate surface, the desired atoms are released. These atoms then diffuse across the surface, find stable nucleation sites, and begin to bond with the substrate and with each other.
This isn't a random splatter; it's an ordered process of nucleation and growth. The film is built layer by layer, resulting in a highly controlled structure, which can be amorphous, polycrystalline, or even single-crystal.
Step 4: Removing the Byproducts
The chemical reactions that deposit the solid film also create unwanted gaseous byproducts. A continuous flow of gas through the chamber, often assisted by a vacuum system, is crucial for desorbing these byproducts from the surface and transporting them away. Failure to do so would contaminate the film and halt the deposition process.
The Defining Outcome: Conformal Coatings
The most significant advantage of the CVD process is its ability to produce highly conformal films. This single characteristic sets it apart from many other deposition techniques.
What "Conformal" Really Means
A conformal coating follows the contours of a surface perfectly, maintaining a uniform thickness everywhere. Imagine dipping a complex object in paint—the paint covers every side, corner, and crevice evenly. This is how CVD behaves.
Because the deposition is driven by a chemical reaction that occurs wherever the precursor gas can reach, it isn't limited by directionality.
The Contrast with PVD
This is in stark contrast to Physical Vapor Deposition (PVD), which is a "line-of-sight" process. In PVD, a material is vaporized and travels in a straight line to the substrate, much like using a can of spray paint. Surfaces facing away from the source receive little to no coating, creating shadows and uneven thickness.
Understanding the Trade-offs
While powerful, CVD is not a universal solution. Its effectiveness is balanced by several important considerations that require expert control.
High Process Temperatures
Traditional thermal CVD often requires temperatures that can damage or alter sensitive substrates, such as plastics or certain electronic components. While plasma-based methods lower this temperature requirement, they introduce the complexity of managing plasma physics.
Precursor Chemistry
The choice of precursor is critical. The chemicals must be volatile enough to be transported as a gas but stable enough not to decompose prematurely. They can also be expensive, highly toxic, or corrosive, demanding sophisticated handling and safety protocols.
Process Complexity
Controlling a CVD process involves a delicate balance of gas flow rates, pressure, temperature, and reaction chemistry. Achieving a repeatable, high-quality film requires precise control over numerous variables, making the equipment and process development more complex than for many PVD techniques.
Making the Right Choice for Your Goal
Selecting a deposition method depends entirely on the requirements of your final product. CVD excels where precision and uniformity are paramount.
- If your primary focus is coating complex 3D shapes or deep trenches uniformly: CVD is the superior choice due to its inherently conformal nature.
- If your primary focus is depositing a high-purity, crystalline film for semiconductors or optics: The atomic-level control of CVD allows for unmatched quality and structural perfection.
- If your primary focus is coating a temperature-sensitive material: A low-temperature variant like Plasma-Enhanced CVD (PECVD) is necessary to avoid damaging the substrate.
- If your primary focus is rapid, simple coating of a flat surface without complex topology: A line-of-sight method like PVD may be a more cost-effective and faster solution.
Understanding that CVD is a process of chemical synthesis, not just physical deposition, is the key to leveraging its unique and powerful capabilities.
Summary Table:
| Feature | Chemical Vapor Deposition (CVD) | Physical Vapor Deposition (PVD) |
|---|---|---|
| Process Type | Chemical reaction & synthesis | Physical transfer (line-of-sight) |
| Coating Uniformity | Highly conformal, even on complex shapes | Directional, can create shadows |
| Typical Temperature | High (lowered in Plasma-Enhanced CVD) | Lower |
| Key Advantage | Uniform, high-purity films on 3D surfaces | Faster for simple, flat surfaces |
Need a high-purity, uniform coating for your complex components?
KINTEK specializes in advanced lab equipment, including CVD systems, to help you achieve precise and reliable thin films for your research or production needs. Our expertise ensures you get the right solution for coating semiconductors, optics, or intricate 3D shapes.
Contact our experts today to discuss how our CVD technology can enhance your laboratory's capabilities!
Visual Guide
Related Products
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Split Chamber CVD Tube Furnace with Vacuum Station Chemical Vapor Deposition System Equipment Machine
- Microwave Plasma Chemical Vapor Deposition MPCVD Machine System Reactor for Lab and Diamond Growth
People Also Ask
- Can plasma enhanced CVD deposit metals? Why PECVD is rarely used for metal deposition
- How do PECVD systems improve DLC coatings on implants? Superior Durability and Biocompatibility Explained
- What is the process of PECVD in semiconductor? Enabling Low-Temperature Thin Film Deposition
- What are different types of thin films? A Guide to Function, Material, and Deposition Methods
- What is the difference between PECVD and APCVD? Choose the Right CVD Method for Your Application



















