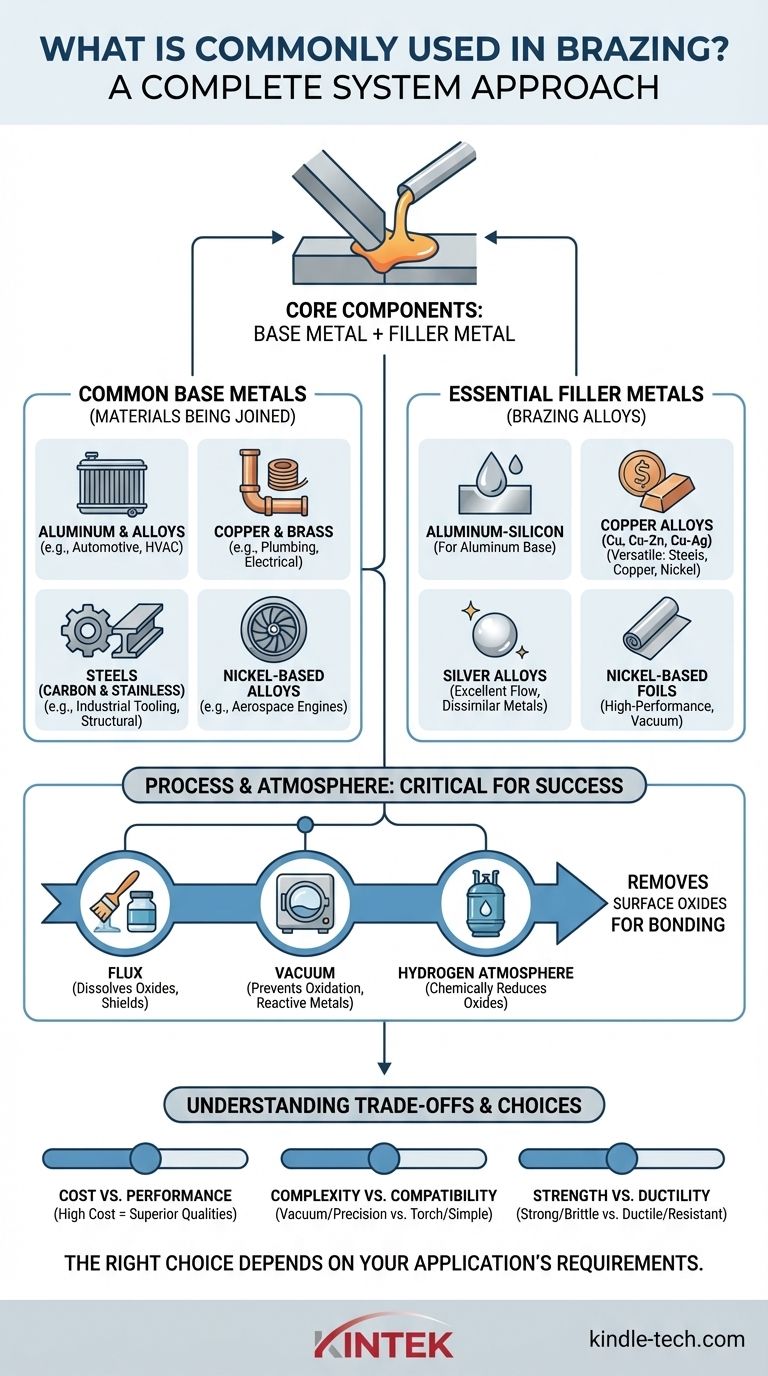
At its core, brazing uses two main components: a base metal, which is the material being joined, and a filler metal, which melts to create the bond. The most common base metals include steel, copper, and aluminum alloys, while filler metals are typically alloys based on aluminum-silicon, copper, or silver.
The key to understanding brazing is to see it as a complete system. The choice of base metal, filler metal, and brazing process are all interconnected, driven by the final application's requirements for strength, corrosion resistance, and cost.
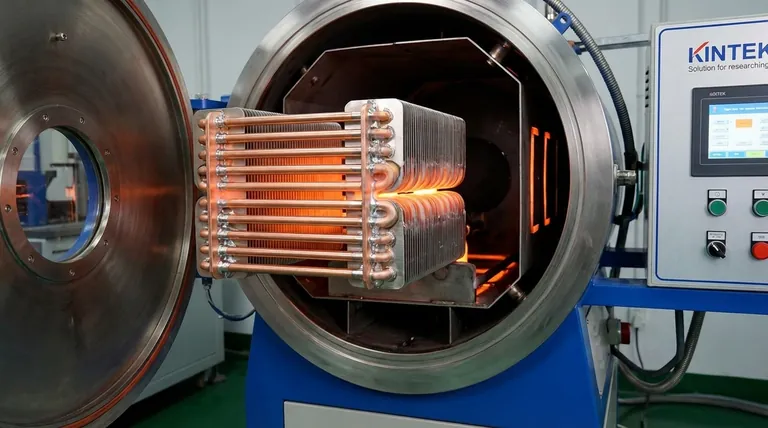
Understanding the Core Components of Brazing
Brazing creates strong, permanent joints between two or more metal parts without melting the parts themselves. This is achieved by heating the assembly and melting a filler metal that flows into the gap between them through capillary action.
Common Base Metals
The base metal is the material you are trying to join. While a vast range of materials can be brazed, a few dominate industrial applications.
- Aluminum and its Alloys: These are extremely common, especially in automotive and HVAC for components like radiators and heat exchangers, due to their light weight and excellent thermal conductivity.
- Copper and Brass: Valued for their high conductivity and corrosion resistance, these are staples in plumbing, electrical, and HVAC industries.
- Steels (Carbon and Stainless): Used for their strength and versatility, steels are brazed for everything from industrial tooling to structural components. Stainless steel is often chosen for its superior corrosion resistance.
- Nickel-Based Alloys (e.g., Inconel): Chosen for high-temperature and high-stress applications, such as in aerospace engines, where performance is critical.
Essential Filler Metals (Brazing Alloys)
The filler metal is the lynchpin of the brazing process. It must have a melting point below that of the base metals and the ability to "wet," or flow over, the surfaces to be joined.
- Aluminum-Silicon (Al-Si): The standard filler for brazing aluminum base metals.
- Copper, Copper-Zinc (Brass), and Copper-Silver: A versatile and widely used family of filler metals for joining steels, copper, and nickel alloys.
- Silver Alloys: These fillers offer excellent flow characteristics and create strong, ductile joints. They are often used for joining dissimilar metals.
- Nickel-Based Foils: Often used in vacuum brazing for high-performance applications, these can include elements like boron, silicon, and phosphorus to control melting characteristics.
The Critical Role of Process and Atmosphere
You cannot select a base metal and filler in isolation. The brazing method directly influences which materials are compatible and how the joint is successfully formed.
The Problem: Surface Oxides
Virtually all metals form a thin, hard layer of oxide on their surface when exposed to air (like rust on steel or the dull layer on aluminum). This oxide layer prevents the filler metal from bonding to the base metal. A successful braze depends entirely on removing it.
Solution 1: Flux
For many common processes like torch or furnace brazing in air, a flux is used. Flux is a chemical compound applied to the joint area that melts before the filler metal, dissolving the oxides and shielding the surface from re-oxidation. Gas-shielded brazing often relies on flux to break the oxide layer on materials like aluminum.
Solution 2: Atmosphere and Alloy Chemistry
In more advanced processes, the environment itself removes the oxides.
- Vacuum Brazing: By removing almost all air from a furnace, oxidation is prevented. For reactive metals like aluminum, specific filler metals (e.g.,
4104series) contain magnesium, which vaporizes at brazing temperature and aggressively breaks down the tough aluminum oxide layer. - Hydrogen Atmosphere Brazing: A pure hydrogen atmosphere can chemically "reduce" (remove) oxides on materials like copper and steel, creating an ultra-clean surface for the filler metal to bond to. However, this process cannot be used for reactive metals like titanium, which form hydrides and become brittle.
Understanding the Trade-offs
Choosing your brazing materials involves balancing competing priorities. There is no single "best" material, only the most appropriate one for the job.
Cost vs. Performance
High-performance filler metals containing silver or gold offer superior joint qualities but come at a significant cost. Similarly, base metals like nickel alloys or titanium are far more expensive than carbon steel or aluminum.
Process Complexity vs. Material Compatibility
Vacuum brazing allows for the joining of complex assemblies and reactive metals like titanium with exceptional precision. However, the equipment and processing time are expensive. Simpler methods like torch brazing are cheaper but are not suitable for sensitive or complex parts.
Joint Strength vs. Ductility
The final properties of the brazed joint depend on the interaction between the filler and base metals. Some filler metals create exceptionally strong joints but may be brittle, while others provide more ductility and fatigue resistance. This choice is critical in parts that experience vibration or thermal cycling.
Making the Right Choice for Your Application
Your material selection should always be guided by the intended purpose of the final product.
- If your primary focus is general-purpose, cost-effective joining: Use common base metals like carbon steel or copper with a standard copper-zinc or silver-based filler metal.
- If your primary focus is lightweight components for heat transfer: Aluminum base metals joined with an aluminum-silicon filler alloy using controlled atmosphere or vacuum brazing is the industry standard.
- If your primary focus is maximum strength and performance in extreme environments: Nickel-based alloys, stainless steels, or titanium brazed in a high-purity vacuum furnace will provide the necessary joint integrity and cleanliness.
Ultimately, successful brazing is achieved by designing a compatible system of base metal, filler metal, and process tailored to your specific goal.
Summary Table:
| Component | Common Examples | Key Characteristics |
|---|---|---|
| Base Metals | Aluminum Alloys, Copper, Steel, Nickel Alloys | Strength, conductivity, corrosion resistance, high-temp performance |
| Filler Metals | Aluminum-Silicon, Copper Alloys, Silver Alloys, Nickel-Based Foils | Melting point below base metal, flows via capillary action |
| Process/Atmosphere | Flux, Vacuum, Hydrogen Atmosphere | Removes oxides to enable filler metal bonding |
Ready to achieve strong, reliable brazed joints for your laboratory or manufacturing needs? The right equipment is critical for success. KINTEK specializes in high-performance lab furnaces, including vacuum and atmosphere brazing systems, designed for precise temperature control and oxide-free results. Whether you're working with aluminum heat exchangers, copper electrical components, or high-strength nickel alloys, our expertise ensures your brazing process is optimized for quality and efficiency. Contact our brazing experts today to discuss your specific application and how KINTEK's equipment can enhance your results.
Visual Guide
Related Products
- Vacuum Heat Treat Sintering Brazing Furnace
- Molybdenum Vacuum Heat Treat Furnace
- 2200 ℃ Graphite Vacuum Heat Treat Furnace
- 1700℃ Laboratory Quartz Tube Furnace with Alumina Tube Tubular Furnace
- Vacuum Heat Treat Furnace with Ceramic Fiber Liner
People Also Ask
- What is the process of a vacuum furnace? Achieve Purity and Precision in High-Temp Processing
- What is the difference between welding and vacuum brazing? Choose the Right Joining Method for Your Project
- What is the cost of a vacuum brazing furnace? A guide to key factors and investment strategy
- What are the different types of brazing welding? A Guide to Choosing the Right Heat Source
- What is a vacuum furnace used for? Unlock Purity in High-Temperature Processing



















