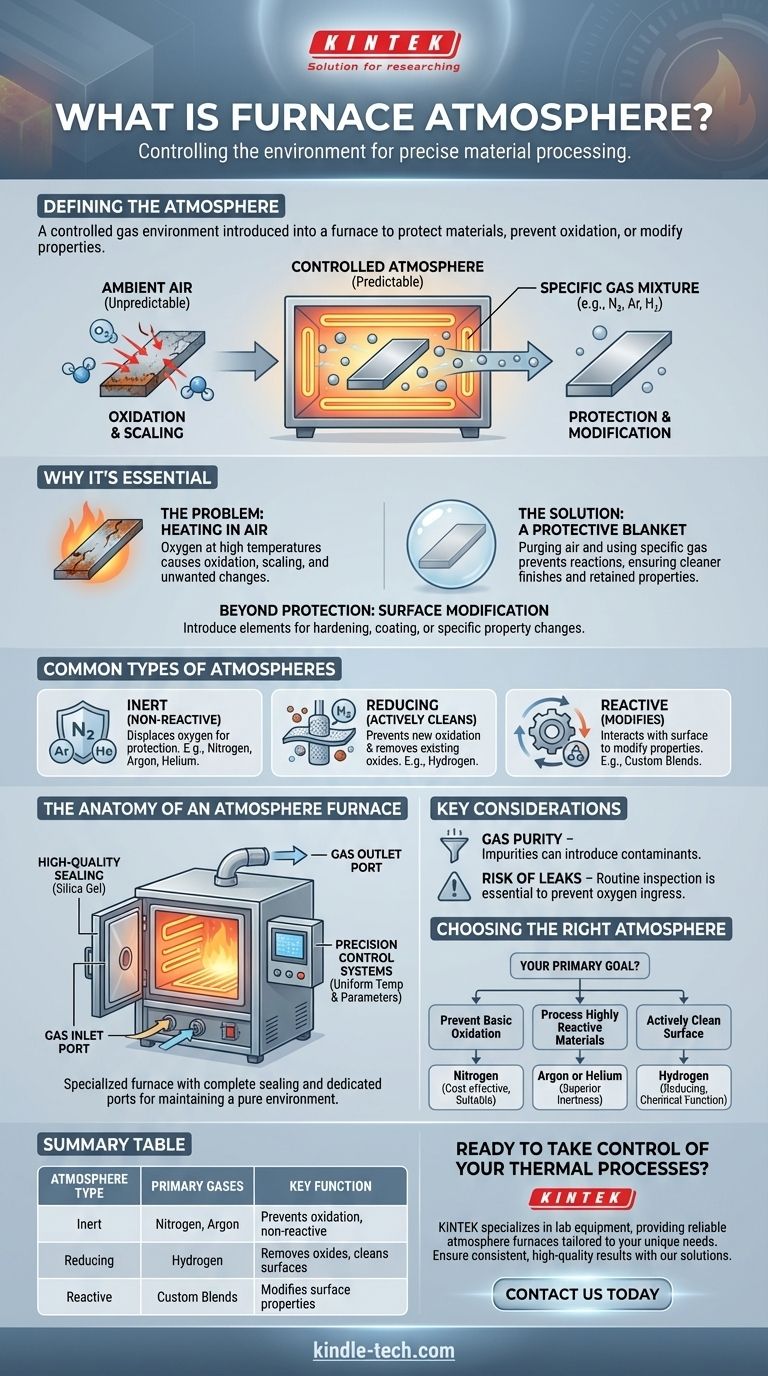
In simple terms, a furnace atmosphere is the specific gas or mixture of gases intentionally introduced into a furnace during a heating process. Instead of heating a material in normal air, this controlled environment is used to protect the material's surface, prevent unwanted chemical reactions like oxidation, or deliberately introduce new elements to modify its properties.
The core purpose of a furnace atmosphere is to replace the unpredictable and reactive nature of ambient air with a controlled, predictable gas environment. This gives engineers and scientists precise control over the final outcome of any thermal process.

Why a Controlled Atmosphere is Essential
Heating materials to high temperatures can fundamentally change them. Controlling the gaseous environment is often just as critical as controlling the temperature itself.
The Problem with Heating in Air
Normal air is about 21% oxygen. When most materials get hot, this oxygen eagerly reacts with their surface.
This reaction, known as oxidation, can cause undesirable effects like scaling, discoloration, and a change in the material's structural or electrical properties.
The Solution: A Protective Blanket
A controlled furnace atmosphere acts as a protective blanket. By purging the furnace chamber of air and filling it with a specific gas, you can eliminate the oxygen and prevent these unwanted reactions.
The result is a cleaner finish and a material that retains its intended characteristics after heating.
Beyond Protection: Surface Modification
Some atmospheres are not just protective; they are intentionally reactive.
In these processes, the gas is chosen specifically to interact with the material's surface. This allows for the introduction of new elements to harden, coat, or otherwise modify the surface in a highly controlled manner.
Common Types of Furnace Atmospheres
The gas chosen depends entirely on the material being processed and the desired outcome. Different gases serve distinct purposes.
Inert Atmospheres
The goal of an inert atmosphere is to be completely non-reactive. Gases like nitrogen, argon, and helium are used to simply displace oxygen and protect the material.
The choice between them often comes down to cost and reactivity at extreme temperatures. While nitrogen is common, argon is even more inert and used for highly sensitive materials.
Reducing Atmospheres
A reducing atmosphere, typically containing hydrogen, goes a step further than an inert one.
Not only does it prevent new oxidation from occurring, but it can also actively strip oxygen atoms from existing oxides on the material's surface. This essentially cleans the part while it is being heated.
The Anatomy of an Atmosphere Furnace
A standard oven cannot maintain a controlled atmosphere. A specialized atmosphere furnace is required and is defined by several key features.
The Importance of Sealing
To maintain a pure internal environment, the furnace must be completely sealed from the outside air.
This is achieved with high-quality welds and high-temperature-resistant seals, often made of silica gel, on all doors and ports. A good seal is the first line of defense against contamination.
Gas Inlet and Outlet Ports
These furnaces have dedicated ports to introduce the desired atmospheric gas and to vent the displaced air. This allows for a complete purge of the chamber before the heating process begins.
Precision Control Systems
Atmosphere furnaces are equipped with systems that ensure uniform temperature and stable control over all process parameters. This precision is critical for achieving consistent, repeatable results.
Understanding the Key Considerations
While powerful, working with controlled atmospheres requires attention to detail. Overlooking these factors can compromise the entire process.
The Challenge of Gas Purity
The effectiveness of the atmosphere is directly tied to the purity of the gas being used. Even small impurities in the gas supply can introduce contaminants that react with the material.
The Critical Risk of Leaks
A small, undetected leak can continuously allow oxygen from the outside air to enter the furnace, defeating the purpose of the controlled atmosphere. Routine inspection and leak detection are essential maintenance tasks.
Safety and Gas Handling
Many gases used in furnace atmospheres present unique safety challenges. Hydrogen, for example, is highly flammable. Proper handling, storage, and ventilation protocols are non-negotiable for ensuring a safe operation.
Choosing the Right Atmosphere for Your Goal
Selecting the correct gas is the most critical decision for any atmospheric thermal process.
- If your primary focus is preventing basic oxidation on common materials: A nitrogen atmosphere is typically the most cost-effective and suitable choice.
- If your primary focus is processing highly reactive materials or ensuring absolute non-reactivity: An argon or helium atmosphere provides a superior level of inertness.
- If your primary focus is actively cleaning a surface by removing existing oxides: A hydrogen-based (reducing) atmosphere is the only option that performs this chemical function.
Ultimately, mastering the furnace atmosphere gives you definitive control over the properties and finish of your materials.
Summary Table:
| Atmosphere Type | Primary Gases | Key Function |
|---|---|---|
| Inert | Nitrogen, Argon | Prevents oxidation, non-reactive |
| Reducing | Hydrogen | Removes existing oxides, cleans surfaces |
| Reactive | Custom Blends | Modifies surface properties (e.g., hardening) |
Ready to take control of your thermal processes? KINTEK specializes in lab equipment and consumables, providing reliable atmosphere furnaces tailored to your laboratory's unique needs. Whether you're working with sensitive materials or require precise surface modifications, our solutions ensure consistent, high-quality results. Contact us today to discuss how we can enhance your lab's capabilities!
Visual Guide
Related Products
- 1200℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1700℃ Controlled Atmosphere Furnace Nitrogen Inert Atmosphere Furnace
- 1400℃ Controlled Atmosphere Furnace with Nitrogen and Inert Atmosphere
- Controlled Nitrogen Inert Hydrogen Atmosphere Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is meant by inert atmosphere? A Guide to Preventing Oxidation & Ensuring Safety
- What is an example of an inert atmosphere? Discover the Best Gas for Your Process
- Can nitrogen gas be heated? Leverage Inert Heat for Precision and Safety
- What is the purpose of inert atmosphere? A Guide to Protecting Your Materials and Processes
- What provides an inert atmosphere? Achieve Safety and Purity with Nitrogen, Argon, or CO2



















