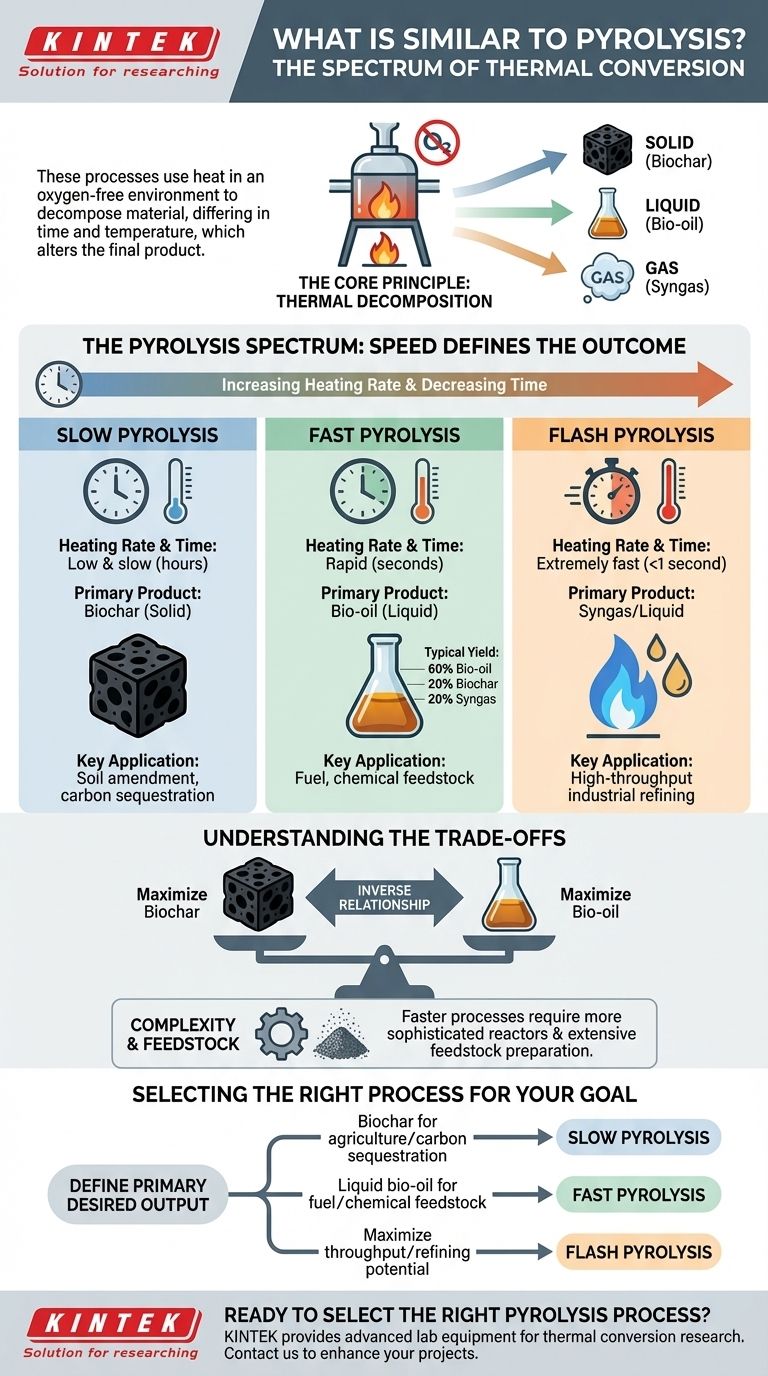
When evaluating thermal conversion technologies, the processes most similar to pyrolysis are actually distinct variations of pyrolysis itself. These methods all use heat in an oxygen-free environment to decompose material, but they differ critically in processing time, temperature, and heating rate, which fundamentally alters the final products.
The core principle to understand is that pyrolysis isn't a single method but a spectrum. The "speed" of the process—slow, fast, or flash—is the primary variable that determines whether the output is predominantly a solid (biochar), a liquid (bio-oil), or a gas (syngas).

The Core Principle: Thermal Decomposition
What is Pyrolysis?
Pyrolysis is the thermal decomposition of organic material at high temperatures in an inert atmosphere, meaning an environment without oxygen.
Because there is no oxygen, the material does not combust. Instead, its chemical compounds break down into a combination of solid, liquid, and gaseous products.
The Pyrolysis Spectrum: Speed Defines the Outcome
The most significant differences between similar thermal processes emerge from the rate of heating and the total processing time.
Slow Pyrolysis: Maximizing Solid Biochar
Slow pyrolysis involves heating feedstock over several hours at relatively lower temperatures. This extended processing time allows for the maximum conversion of the material into biochar, a stable, carbon-rich solid.
This method is often preferred when the primary goal is to create a soil amendment, a filtration medium, or a means of carbon sequestration.
Fast Pyrolysis: A Focus on Liquid Bio-oil
Fast pyrolysis is the most common industrial approach. It heats material very quickly, completing the entire process in just a few seconds.
This rapid conversion minimizes the formation of char and maximizes the production of a liquid known as bio-oil, which can be used as a fuel or upgraded into other chemicals. A typical yield is 60% bio-oil, 20% biochar, and 20% syngas.
Flash Pyrolysis: Pushing for Maximum Yield and Throughput
Flash pyrolysis represents an even more extreme version of fast pyrolysis, with extremely high heating rates and very short residence times (often less than a second).
The primary advantage is the potential for higher yields of the desired liquid or gaseous products. Its outputs are often considered a better-quality feedstock for subsequent refining processes.
Understanding the Trade-offs
Choosing a pyrolysis method is not about finding the "best" one, but the one best suited for a specific goal, as each involves clear trade-offs.
Goal Dictates the Process
The fundamental trade-off is between the desired products. There is an inverse relationship between biochar and bio-oil yields.
Processes designed to maximize biochar (slow pyrolysis) will inherently produce less bio-oil, and vice-versa for fast and flash pyrolysis.
Complexity and Feedstock Requirements
Faster processes like fast and flash pyrolysis generally require more sophisticated and precisely engineered reactors to manage the high heat transfer rates.
They also often demand more extensive feedstock preparation, such as grinding materials into very fine particles to ensure rapid and complete heating. This can increase both capital and operational costs.
Selecting the Right Process for Your Goal
To make an informed decision, you must first define your primary desired output.
- If your primary focus is producing biochar for agriculture or carbon sequestration: Slow pyrolysis is the most direct and effective method.
- If your primary focus is generating liquid bio-oil for fuel or chemical feedstock: Fast pyrolysis offers a balanced, proven, and widely implemented solution.
- If your primary focus is maximizing throughput and industrial refining potential: Flash pyrolysis provides the highest performance for liquid and gas yields, though potentially with greater complexity.
Ultimately, understanding this spectrum empowers you to select the precise thermal conversion tool for your specific material and objective.
Summary Table:
| Pyrolysis Type | Heating Rate & Time | Primary Product | Key Application |
|---|---|---|---|
| Slow Pyrolysis | Low & slow (hours) | Biochar (solid) | Soil amendment, carbon sequestration |
| Fast Pyrolysis | Rapid (seconds) | Bio-oil (liquid) | Fuel, chemical feedstock |
| Flash Pyrolysis | Extremely fast (<1 second) | Syngas/Liquid | High-throughput industrial refining |
Ready to select the right pyrolysis process for your specific goals?
At KINTEK, we specialize in providing advanced lab equipment and consumables for thermal conversion research and development. Whether you're focused on biochar production, bio-oil refinement, or syngas optimization, our expertise and high-quality solutions can help you achieve precise and reliable results.
Contact us today to discuss how our equipment can enhance your pyrolysis projects and drive your laboratory's success. Get in touch with our experts now!
Visual Guide
Related Products
- Customizable Laboratory High Temperature High Pressure Reactors for Diverse Scientific Applications
- Wall Mounted Water Distillation Unit
- Desktop Fast Laboratory Autoclave Sterilizer 35L 50L 90L for Lab Use
- Inclined Rotary Plasma Enhanced Chemical Vapor Deposition PECVD Equipment Tube Furnace Machine
- High Temperature Constant Temperature Heating Circulator Water Bath Chiller Circulator for Reaction Bath
People Also Ask
- What are some ways you can prevent injury when dealing with hot substances and objects? A Proactive Framework for Thermal Safety
- What are the five basic heat treatment processes? A Guide to Metal Hardening & Tempering
- Is biochar production sustainable? Unlocking True Carbon Sequestration and Soil Health
- What is target poisoning in sputtering? A Guide to Process Instability and Control
- What is the difference between pyrolysis? Slow vs. Fast vs. Flash Explained
- Can pyrolysis generate electricity? Unlock Power from Waste and Biomass
- Is sintering environmentally friendly? Balancing Energy Use with Material Efficiency
- What are the advantages of selective heat sintering? Achieve Complex, Support-Free 3D Printed Parts



















