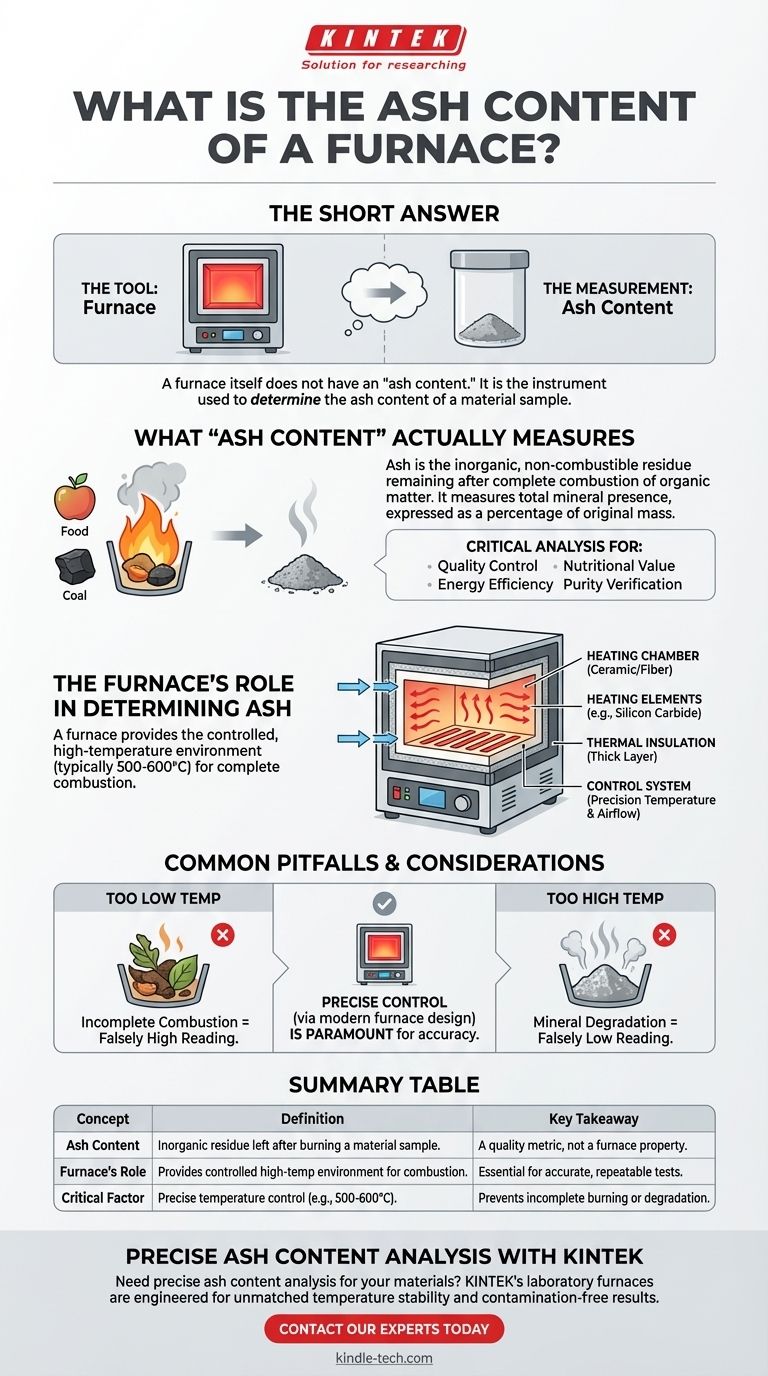
The short answer is this: A furnace itself does not have an "ash content." Rather, a furnace is the instrument used to determine the ash content of a material sample. Ash content is a measurement of the inorganic, non-combustible residue left after a substance is completely burned at high temperatures.
The core misunderstanding is between the tool and the measurement. A furnace provides the controlled, high-temperature environment necessary to perform an ashing test, which measures the incombustible mineral content within a sample like food, coal, or plastic.

What "Ash Content" Actually Measures
Defining the Metric
Ash is the inorganic residue remaining after the complete combustion of organic matter. The "ash content" is therefore a measure of the total amount of minerals present within a material.
This value is expressed as a percentage of the original sample's mass. It is a critical indicator of quality, purity, and composition in many industries.
Why It's a Critical Analysis
Measuring ash content is fundamental for quality control. For example, in the food industry, it helps determine nutritional value. In the coal industry, high ash content means lower energy efficiency and more waste.
In materials science, it can verify the purity of polymers or other composites by measuring the amount of inorganic filler material.
The Furnace's Role in Determining Ash
The Principle of High-Temperature Combustion
To measure ash, all combustible (organic) parts of a sample must be burned away, leaving only the stable inorganic minerals behind. A laboratory furnace is designed to do exactly this.
The process involves placing a precisely weighed sample into the furnace and heating it to a specific, high temperature (typically 500-600°C or higher) for a set period, ensuring complete combustion.
Key Furnace Components for the Task
While designs vary, furnaces used for ashing share several critical components that create a stable and controlled environment:
- A Heating Chamber: This is the core of the furnace, typically made of high-temperature alumina or quartz ceramic fiber. It is built to withstand extreme heat and prevent chemical reactions with the sample.
- Heating Elements: Coils or rods made of materials like silicon carbide or molybdenum are embedded in the chamber walls. They generate the high temperatures required for combustion.
- Thermal Insulation: A thick layer of insulation surrounds the heating chamber. This minimizes energy loss, ensures temperature uniformity, and keeps the outer shell safe to touch.
- A Control System: This is the brain of the operation. It uses a thermocouple to measure the internal temperature and adjusts power to the heating elements to maintain a precise, stable setpoint.
Common Pitfalls and Key Considerations
Temperature Control is Paramount
The accuracy of an ash content test depends entirely on precise temperature control. If the temperature is too low, combustion will be incomplete, leaving behind unburned organic matter and leading to an inaccurate, falsely high reading.
Conversely, if the temperature is too high, some minerals can degrade or volatilize, leading to a falsely low reading. Standard testing methods (like ASTM or ISO) specify exact temperatures for this reason.
Ensuring Complete Combustion
Proper airflow is necessary to supply the oxygen required for a clean and complete burn. Modern ashing furnaces, often called muffle furnaces, are designed with vents to allow for controlled air exchange.
Preventing Sample Contamination
The materials used to construct the furnace chamber are critical. Refractory ceramics like alumina and quartz are used because they are chemically inert at high temperatures and will not shed particles that could contaminate the sample and skew the results.
Making the Right Choice for Your Analysis
To perform this analysis correctly, you must match the instrument to the goal.
- If your primary focus is routine quality control (e.g., food, feed, wastewater): A standard muffle furnace with a programmable controller is the essential tool for reliable and repeatable ashing.
- If your primary focus is advanced materials research: A tube furnace might be required if you need to control the atmosphere (e.g., performing the test in an inert gas or under a vacuum) for specialized thermal analysis.
Understanding the distinction between the measurement and the method is the first step toward accurate and meaningful material analysis.
Summary Table:
| Concept | Definition | Key Takeaway |
|---|---|---|
| Ash Content | The inorganic residue left after burning a material sample. | A quality metric, not a property of the furnace itself. |
| Furnace's Role | Provides controlled high-temperature environment for complete combustion. | Essential for accurate, repeatable ashing tests. |
| Critical Factor | Precise temperature control (typically 500-600°C). | Prevents incomplete burning or mineral degradation. |
Need precise ash content analysis for your materials? KINTEK's laboratory furnaces are engineered for unmatched temperature stability and contamination-free results, ensuring your quality control and research data is accurate and reliable. Whether you work with food, coal, plastics, or advanced composites, our equipment is built to meet rigorous standards. Contact our experts today to find the perfect furnace for your application!
Visual Guide
Related Products
- 1700℃ Muffle Oven Furnace for Laboratory
- 1800℃ Muffle Oven Furnace for Laboratory
- 1400℃ Muffle Oven Furnace for Laboratory
- Laboratory Muffle Oven Furnace Bottom Lifting Muffle Furnace
- High Temperature Muffle Oven Furnace for Laboratory Debinding and Pre Sintering
People Also Ask
- How does an Industrial Box Resistance Furnace contribute to the long-term thermal exposure experiments of GH3535 alloy?
- How does a laboratory muffle furnace ensure accuracy in biomass ash determination? Optimize Your Material Analysis
- How do you adjust the temperature on a muffle furnace? Master Precise Control for Your Lab
- What is the maximum temperature of muffle furnace? A Guide from 1100°C to 1800°C
- Where is a muffle furnace used? Essential for Clean, High-Temperature Processing
- How does a high-temperature muffle furnace function during the preparation of LATP solid electrolyte ceramic sheets?
- What are the hazards of a muffle furnace? Understanding the Critical Risks for Lab Safety
- How do you determine the ash content of a food sample? Choose the Right Method for Accurate Mineral Analysis



















