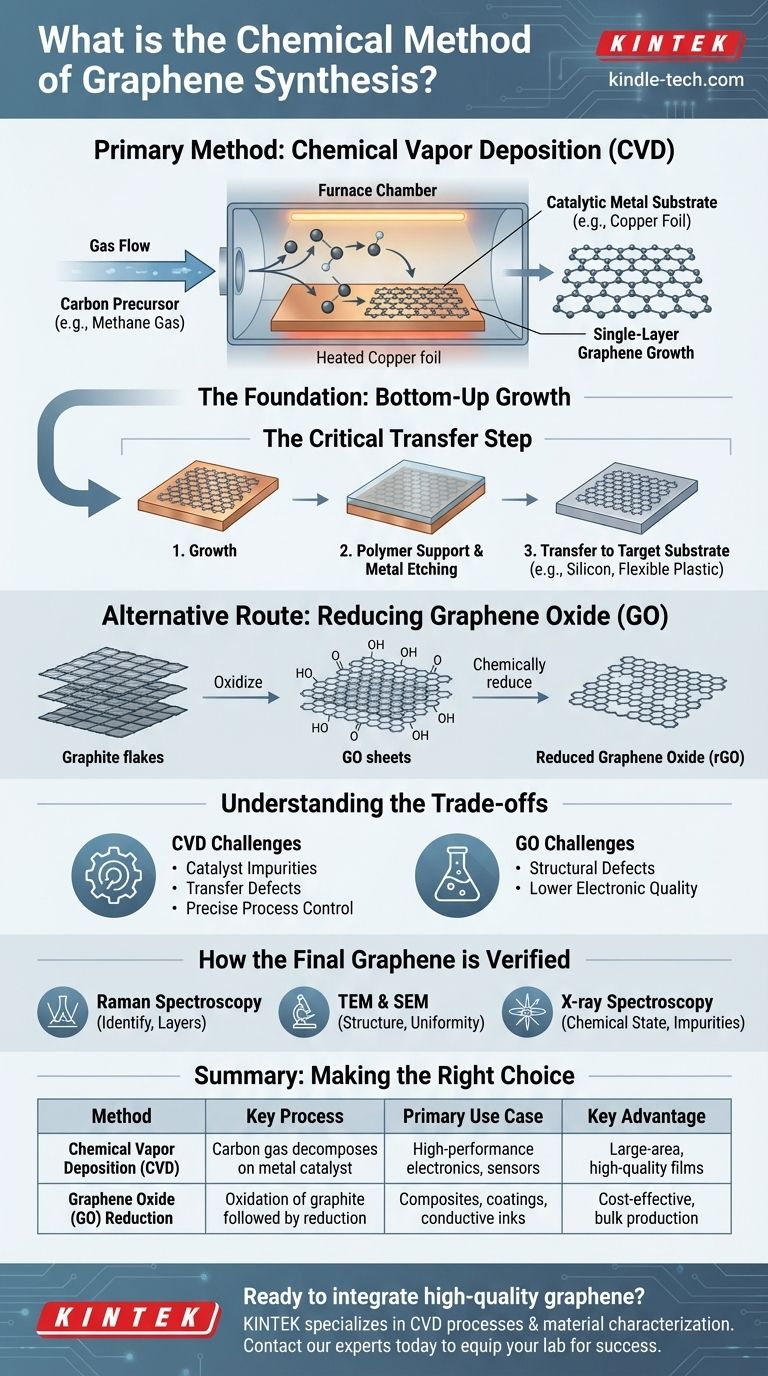
The primary chemical method for synthesizing high-quality, large-area graphene is Chemical Vapor Deposition (CVD). This "bottom-up" technique involves introducing a carbon-containing gas, such as methane, into a high-temperature chamber where it decomposes on a catalytic metal substrate, typically copper foil. The carbon atoms then reassemble into a continuous, single-atomic layer of graphene across the substrate's surface. Another important chemical route involves the reduction of graphene oxide.
While mechanical exfoliation produces the highest quality graphene flakes, it is not scalable. Chemical synthesis methods, particularly CVD, are the most viable pathway for producing the large, uniform graphene sheets necessary for commercial electronic and industrial applications.

The Foundation of Chemical Synthesis: Bottom-Up Growth
Chemical synthesis is fundamentally a "bottom-up" approach. Instead of carving a small piece from a larger block (like mechanical exfoliation from graphite), you are building graphene atom by atom from a chemical precursor.
What is Chemical Vapor Deposition (CVD)?
CVD is a process that deposits a solid material from a gaseous phase onto a substrate. For graphene, this means a carbon-source gas is heated until it breaks down.
These newly freed carbon atoms then diffuse and arrange themselves on a catalytic metal surface, forming the characteristic hexagonal lattice of graphene. The process requires precise control over temperature, gas flow, and pressure.
The Key Ingredients: Precursors and Catalysts
The success of CVD depends entirely on its inputs.
The most common carbon source, or precursor, is methane gas due to its simple structure and clean decomposition. Other sources like petroleum asphalt are cheaper but introduce more complexity and potential impurities.
A catalyst is essential to facilitate the reaction at lower temperatures. Metal foils, such as copper (Cu) and nickel (Ni), are widely used both as catalysts and as the growth substrate. The choice of catalyst influences the quality and number of graphene layers formed.
The Critical Transfer Step
After growth, the graphene sheet sits on the metal foil. To be used in any application, it must be transferred to a target substrate, like silicon or flexible plastic.
This delicate process typically involves coating the graphene with a polymer support, etching away the metal catalyst, and then "stamping" the graphene/polymer film onto the new substrate before dissolving the support.
An Alternative Chemical Route: Reducing Graphene Oxide (GO)
Another major chemical method starts with inexpensive graphite. The graphite is aggressively oxidized to form graphene oxide (GO), a material rich in oxygen-containing functional groups.
This GO is easily dispersed in water, forming single-layer sheets. These sheets are then exposed to chemical reducing agents that remove the oxygen, yielding reduced Graphene Oxide (rGO). While this method is excellent for producing large quantities of graphene-like material for composites and inks, the resulting rGO often has more structural defects than CVD-grown graphene.
Understanding the Trade-offs
No synthesis method is perfect. Chemical approaches offer scalability but come with inherent challenges that are critical to understand.
The Challenge of Quality and Purity
The catalysts used in CVD, such as nickel or iron, can sometimes leave behind metallic impurities on the graphene sheet.
The process required to remove the catalyst after growth, or to transfer the graphene to a new substrate, can introduce tears, wrinkles, and other structural defects that compromise its exceptional electronic and mechanical properties.
The Difficulty of Process Control
CVD is not a simple recipe. Achieving a uniform, single-layer sheet over a large area requires meticulous control over gas transport kinetics and reaction temperature.
Even minor fluctuations can lead to the growth of undesirable multi-layer patches or an increase in defects, impacting the final material's performance and consistency.
How the Final Graphene is Verified
Once synthesized, the material must be analyzed to confirm its quality. Several techniques are essential for characterization.
Identifying Graphene: Raman Spectroscopy
Raman spectroscopy is the gold standard for identifying graphene and determining the number of layers. It provides a fast, non-destructive spectral fingerprint that confirms the material's structure.
Examining Structure and Defects: TEM & SEM
Transmission Electron Microscopy (TEM) and Scanning Electron Microscopy (SEM) provide direct visual evidence of the graphene. TEM reveals fine details of the atomic lattice, while SEM is used to examine the surface topography and film uniformity over larger areas.
Confirming Chemical State: X-ray Spectroscopy
X-ray spectroscopy is used to characterize the chemical states within the sample, helping to identify any impurities or residual oxygen groups (especially important for rGO) that could affect performance.
Making the Right Choice for Your Goal
The "best" chemical synthesis method depends entirely on the intended application.
- If your primary focus is high-performance electronics: CVD is the preferred method because it produces large-area, high-quality films with superior electronic properties.
- If your primary focus is bulk production for composites, coatings, or inks: The chemical reduction of graphene oxide is more scalable and cost-effective for applications where perfect atomic structure is less critical than quantity.
- If your primary focus is fundamental research on pristine material: Mechanical exfoliation remains the benchmark for producing flawless, but very small, graphene flakes for scientific study.
Ultimately, mastering chemical synthesis is the bridge between graphene's theoretical promise and its real-world application.
Summary Table:
| Method | Key Process | Primary Use Case | Key Advantage |
|---|---|---|---|
| Chemical Vapor Deposition (CVD) | Carbon gas decomposes on metal catalyst (e.g., copper) | High-performance electronics, sensors | Large-area, high-quality films |
| Graphene Oxide (GO) Reduction | Oxidation of graphite followed by chemical reduction | Composites, coatings, conductive inks | Cost-effective, bulk production |
Ready to integrate high-quality graphene into your research or product development? The right synthesis method is critical to your success. KINTEK specializes in providing the advanced lab equipment and consumables necessary for precise CVD processes and material characterization. Our expertise supports laboratories in achieving consistent, high-quality graphene synthesis. Contact our experts today to discuss how we can equip your lab for success.
Visual Guide
Related Products
- Chemical Vapor Deposition CVD Equipment System Chamber Slide PECVD Tube Furnace with Liquid Gasifier PECVD Machine
- Customer Made Versatile CVD Tube Furnace Chemical Vapor Deposition Chamber System Equipment
- RF PECVD System Radio Frequency Plasma-Enhanced Chemical Vapor Deposition RF PECVD
- Vacuum Hot Press Furnace Heated Vacuum Press Machine Tube Furnace
- Vertical Laboratory Tube Furnace
People Also Ask
- What is the LPCVD technique? Achieve High-Purity, Uniform Thin Films for Semiconductors
- What material is use for coating on carbides? Boost Tool Life & Performance with the Right Coating
- Which one of the following methods is used to make a thin film? A Guide to PVD vs. CVD
- What is the general process of growing diamonds using the CVD method? Master Precision Lab-Grown Diamond Technology
- What are the applications of sputter deposition? Achieve Superior Thin Films for Electronics and Optics
- What are the typical characteristics of crystals grown by the CVD method? Key Insights into Shape, Color, and Clarity
- How do Chemical Vapor Deposition (CVD) systems ensure material quality? Precision Control for Graphene-Coated Electrodes
- What are the advantages and disadvantages of LPCVD? Mastering High-Quality Thin Film Deposition



















