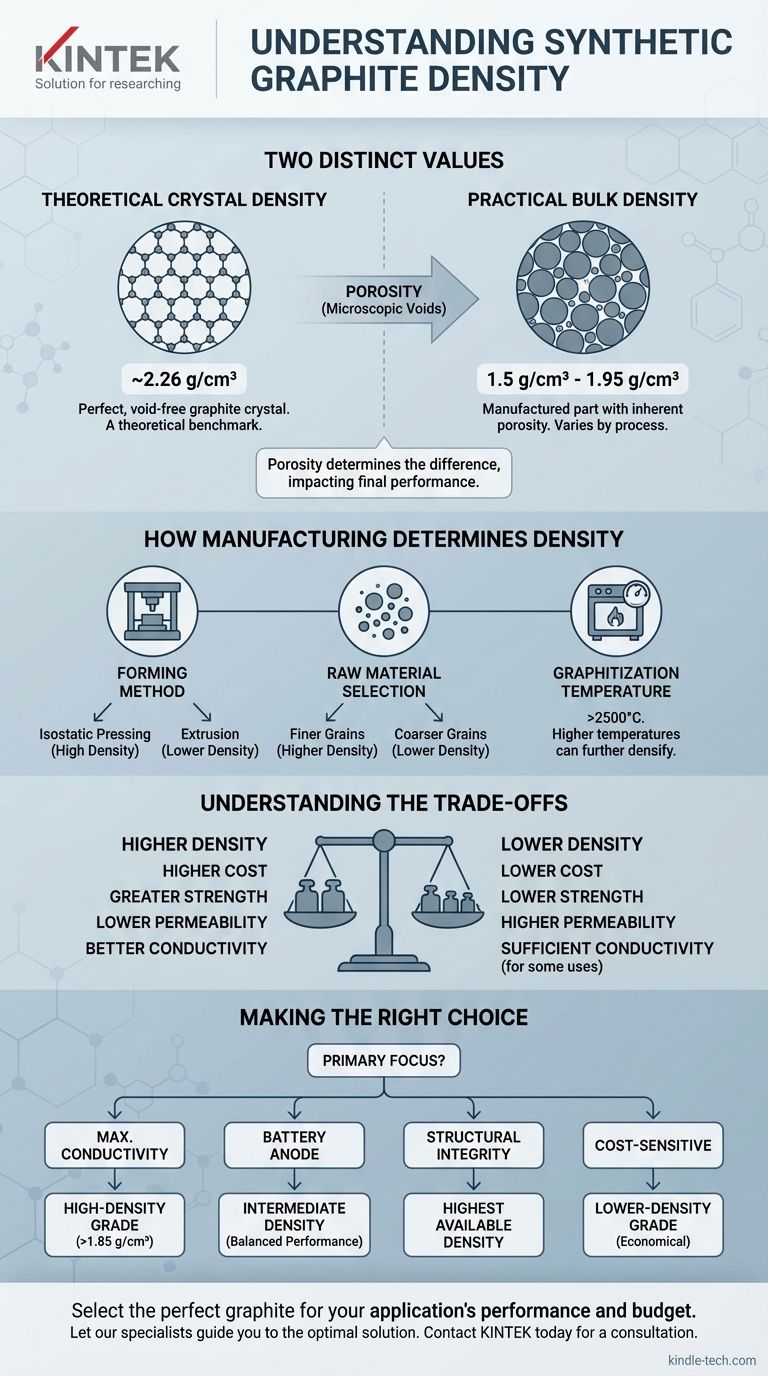
The density of synthetic graphite is best understood as two distinct values. The theoretical, or true, density of a perfect graphite crystal is approximately 2.26 g/cm³. However, the practical bulk density of a manufactured synthetic graphite part is almost always lower, typically ranging from 1.5 g/cm³ to 1.95 g/cm³ due to inherent porosity from the production process.
The key is to distinguish between the ideal density of the graphite crystal and the practical bulk density of a finished product. This difference is almost entirely due to the microscopic void spaces, or porosity, created during manufacturing, which directly impacts the material's final performance.

Why Density Isn't a Single Number
The discrepancy between the theoretical and practical density of synthetic graphite is not an imperfection; it is a fundamental characteristic of the material that is controlled to achieve specific properties for different applications.
The Theoretical Limit: Crystal Density
A perfect graphite crystal consists of carbon atoms arranged in a hexagonal lattice structure.
Based on the atomic weight of carbon and the spacing of these crystal planes, the absolute maximum density is calculated to be ~2.26 g/cm³. This value represents a solid, void-free material and serves as an important theoretical benchmark.
The Practical Reality: Bulk Density
Real-world synthetic graphite is made by combining a carbon aggregate (like petroleum coke) with a binder (like coal tar pitch), pressing it into a shape, and then heat-treating it at extreme temperatures.
This process inevitably leaves microscopic voids, or pores, between the original particles. These pores lower the overall mass per unit volume, resulting in a bulk density that is less than the theoretical maximum.
The Critical Role of Porosity
Porosity is the measure of the empty space within a material, usually expressed as a percentage.
It has an inverse relationship with bulk density: as porosity increases, bulk density decreases. Engineers intentionally control porosity to influence properties like permeability, machinability, and performance in battery applications.
How Manufacturing Determines Density
The final density of a synthetic graphite component is not an accident. It is a direct result of precise decisions made throughout the entire manufacturing process.
Forming Method
The method used to shape the material before baking is a primary factor.
Isostatic pressing, which applies very high pressure uniformly from all directions, produces a very dense, fine-grained graphite with low porosity. Extrusion, by contrast, typically results in a lower-density material.
Raw Material Selection
The size and type of the initial carbon particles (the aggregate) play a crucial role.
Using finer grain aggregates generally allows for better packing, reducing the space between particles and leading to a higher final density after processing.
Graphitization Temperature
The final step involves heating the material to temperatures above 2500°C to create the ordered graphitic crystal structure.
Higher graphitization temperatures can help further densify the material, bringing its final bulk density closer to the theoretical limit, though the effect is secondary to the forming method.
Understanding the Trade-offs
Selecting a specific density is an engineering compromise. Optimizing for one property often means accepting a limitation in another.
Density vs. Cost
Achieving higher density requires more intensive processing, such as isostatic pressing and the use of premium raw materials. Consequently, higher-density graphite is almost always more expensive.
Density vs. Strength
Bulk density is directly correlated with mechanical properties. A denser graphite part will have higher compressive strength and be more resistant to wear and erosion.
Density vs. Permeability
Porosity dictates how easily gases or liquids can pass through the graphite. High-density, low-porosity graphite is required for applications needing a tight seal, such as in crucibles or nuclear reactors.
Making the Right Choice for Your Application
Your choice of synthetic graphite density should be driven entirely by the primary requirements of your project.
- If your primary focus is maximum electrical or thermal conductivity: Select a high-density grade (e.g., >1.85 g/cm³), as this ensures more pathways for electrons and heat to travel.
- If your primary focus is battery anode performance: A carefully controlled intermediate density is often best, balancing high energy capacity (denser material) with the necessary porosity for electrolyte access and lithium-ion diffusion.
- If your primary focus is structural integrity or wear resistance: Choose the highest density available that fits your budget, as this directly relates to greater mechanical strength.
- If your primary focus is cost-sensitive applications like furnace parts: A lower-density extruded graphite grade is often the most economical and perfectly sufficient choice.
By understanding the link between density, manufacturing, and performance, you can confidently select the precise grade of graphite to meet your technical and financial goals.
Summary Table:
| Density Type | Typical Value (g/cm³) | Key Characteristics |
|---|---|---|
| Theoretical (Crystal) Density | ~2.26 | Density of a perfect graphite crystal; a theoretical maximum. |
| Bulk (Practical) Density | 1.5 - 1.95 | Density of a manufactured part; varies based on porosity and manufacturing process. |
Select the perfect graphite for your application's performance and budget.
Understanding the critical balance between density, porosity, strength, and cost is essential for choosing the right synthetic graphite. Whether you need high-density material for superior conductivity and strength in crucibles or reactors, or a cost-effective grade for furnace components, KINTEK's expertise ensures you get a material tailored to your lab's specific requirements.
Let our specialists guide you to the optimal solution. Contact KINTEK today for a consultation on high-performance lab equipment and consumables.
Visual Guide
Related Products
- Carbon Graphite Plate Manufactured by Isostatic Pressing Method
- Graphite Vacuum Continuous Graphitization Furnace
- Vertical High Temperature Graphite Vacuum Graphitization Furnace
- Ultra-High Temperature Graphite Vacuum Graphitization Furnace
- Large Vertical Graphite Vacuum Graphitization Furnace
People Also Ask
- What is the use of sinter in blast furnace? Optimize Iron Production with Engineered Feedstock
- What is coating deposition? Engineer Superior Surface Properties for Your Materials
- What is a slow pyrolysis? A Guide to Maximizing Biochar Production from Biomass
- What is fusion in XRF? Achieve Unmatched Accuracy in Elemental Analysis
- What technical challenges do integrated membrane technologies address? Overcome Mass Transfer in Wastewater Treatment
- What is biomass conversion efficiency? Maximize Your Bioenergy Output and ROI
- How does adding Alumina or Yttria reduce SiC sintering temperature? Efficient Liquid-Phase Sintering Explained
- Which solvent is normally used in IR spectroscopy? Optimize Your Sample Prep for Clearer Results



















