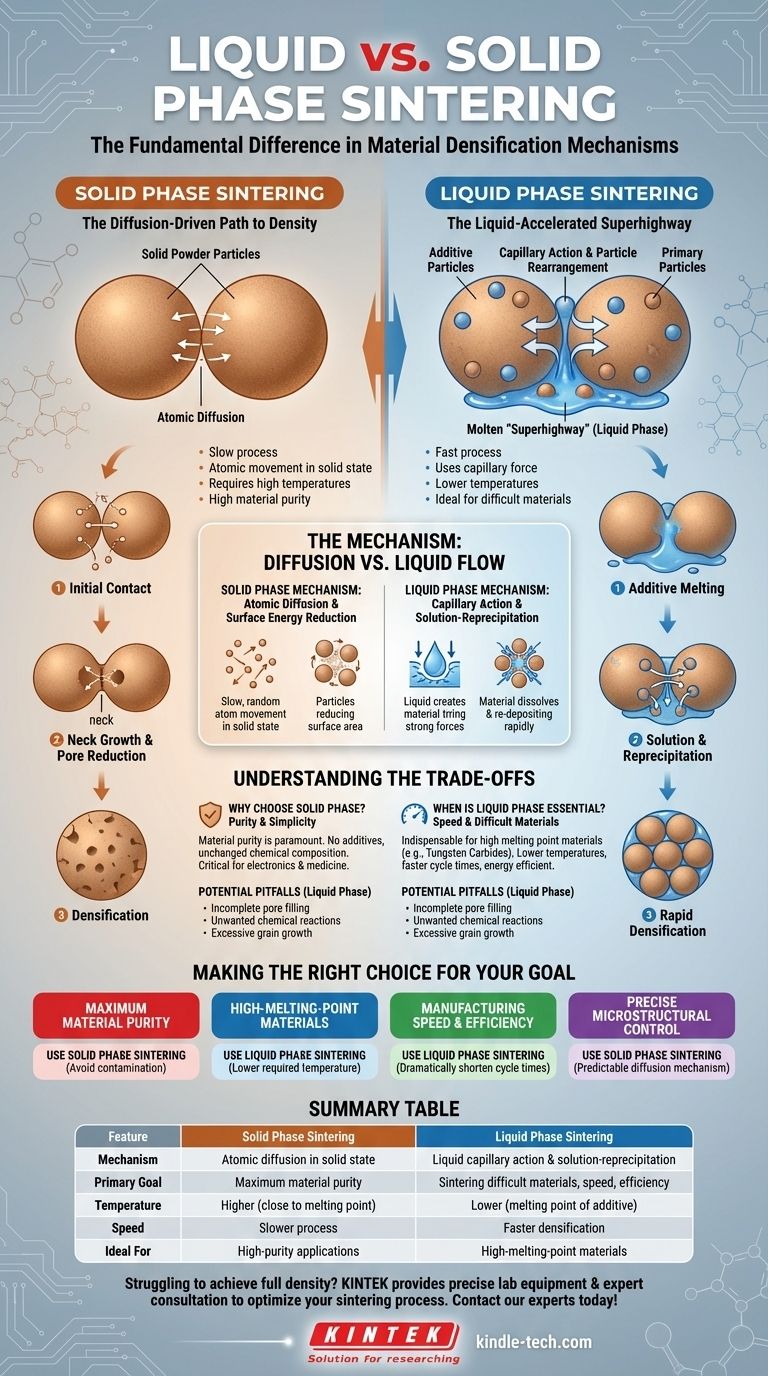
The fundamental difference between liquid and solid phase sintering lies in the state of the materials during the heating process. In solid phase sintering, the entire powder compact remains solid, relying on atomic movement across particle surfaces to bond. Conversely, liquid phase sintering introduces a small amount of an additive that melts, creating a liquid that accelerates the bonding and densification process.
The core distinction is not just the presence of a liquid, but the mechanism it enables. Solid phase sintering is a slow process governed by atomic diffusion, while liquid phase sintering creates a molten "superhighway" that uses capillary force and rapid particle transport to achieve density more quickly and at lower temperatures.
The Mechanism of Solid Phase Sintering
Solid phase sintering is the foundational method for densifying a powder compact without melting the primary material. Its efficiency depends entirely on the movement of atoms in their solid state.
The Foundation: Atomic Diffusion
At high temperatures, atoms in the powder particles become more mobile. They begin to move and diffuse across the boundaries where individual particles touch.
The Goal: Reducing Surface Energy
This atomic movement is driven by a natural tendency to reduce the system's total surface energy. A fine powder has a massive amount of surface area, and by bonding together, the particles form a more stable, lower-energy structure.
The Result: Neck Growth and Pore Reduction
As atoms migrate to the points of contact, they form small bridges, or "necks," between particles. Over time, these necks grow larger, pulling the particles closer together and systematically shrinking the pores or voids between them.
How Liquid Phase Sintering Changes the Game
Liquid phase sintering is an engineered solution to overcome the limitations of the solid phase process, especially for materials that are difficult to sinter.
The Key Ingredient: The Additive
This process begins by mixing the primary powder with a small amount of a secondary powder, often called a sintering aid or binder. This additive is chosen specifically because it has a lower melting point than the main material.
The Liquid's Role: Capillary Action
When the compact is heated to the sintering temperature, the additive melts and flows into the pores between the solid particles. This liquid creates powerful capillary forces that pull the solid particles together, rapidly rearranging them into a much denser packing arrangement.
The Acceleration Factor: Solution and Reprecipitation
The liquid phase acts as a high-speed transport medium. The primary solid particles partially dissolve into the liquid at their points of contact. This dissolved material then travels through the liquid and re-precipitates in the neck regions between particles, efficiently filling voids and dramatically accelerating densification.
Understanding the Trade-offs
Choosing between these two methods involves a clear set of trade-offs related to material properties, process efficiency, and final component requirements.
Why Choose Solid Phase? Purity and Simplicity
Solid phase sintering is the ideal choice when material purity is paramount. Since no additives are introduced, the final component's chemical composition is unchanged. This is critical for applications in electronics, medicine, and research where even trace contaminants are unacceptable.
When is Liquid Phase Essential? Speed and Difficult Materials
This method is indispensable for materials with extremely high melting points or poor atomic diffusion, such as tungsten carbides and many advanced ceramics. The liquid phase allows for sintering at significantly lower temperatures and in much shorter times, making the process more energy-efficient and commercially viable.
Potential Pitfalls of Liquid Phase
The presence of a liquid introduces complexity. There is a risk of incomplete pore filling, unwanted chemical reactions between the liquid and solid particles, or excessive grain growth, which can negatively impact the final mechanical properties of the component.
Making the Right Choice for Your Goal
Your final decision should be guided by the primary objective for your component and manufacturing process.
- If your primary focus is maximum material purity: Use solid phase sintering to avoid any contamination from secondary additives.
- If your primary focus is sintering high-melting-point materials: Use liquid phase sintering to lower the required temperature and make the process feasible.
- If your primary focus is manufacturing speed and energy efficiency: Use liquid phase sintering to dramatically shorten cycle times and reduce costs.
- If your primary focus is precise microstructural control in a single-component system: Use solid phase sintering for its predictable, diffusion-controlled mechanism.
Ultimately, understanding this core difference empowers you to select the most effective and efficient path to achieving full material density.
Summary Table:
| Feature | Solid Phase Sintering | Liquid Phase Sintering |
|---|---|---|
| Mechanism | Atomic diffusion in solid state | Liquid capillary action & solution-reprecipitation |
| Primary Goal | Maximum material purity | Sintering difficult materials, speed, efficiency |
| Temperature | Higher (close to material's melting point) | Lower (melting point of the additive) |
| Speed | Slower process | Faster densification |
| Ideal For | High-purity applications (electronics, medicine) | High-melting-point materials (tungsten carbide, ceramics) |
Struggling to achieve full density with your powder materials? The choice between solid and liquid phase sintering is critical for your component's performance and manufacturing efficiency. KINTEK specializes in providing the precise lab equipment and expert consultation needed to optimize your sintering process. Whether you require high-purity results or need to sinter challenging materials efficiently, we have the solutions. Contact our experts today to discuss how we can enhance your laboratory's capabilities and help you select the perfect sintering path for your goals.
Visual Guide
Related Products
- 2200 ℃ Tungsten Vacuum Heat Treat and Sintering Furnace
- Vacuum Heat Treat Sintering Brazing Furnace
- Vacuum Heat Treat and Molybdenum Wire Sintering Furnace for Vacuum Sintering
- Molybdenum Vacuum Heat Treat Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is vacuum sintering? Achieve Unmatched Purity and Performance for Advanced Materials
- What is the sintering time? A Critical Process Variable for Material Density and Strength
- Does sintering use diffusion? The Atomic Mechanism for Building Stronger Materials
- Why would you braze instead of solder? For Superior Joint Strength and High-Temperature Performance
- What are the methods of brazing heating? Choose the Right Method for Your Production Needs



















