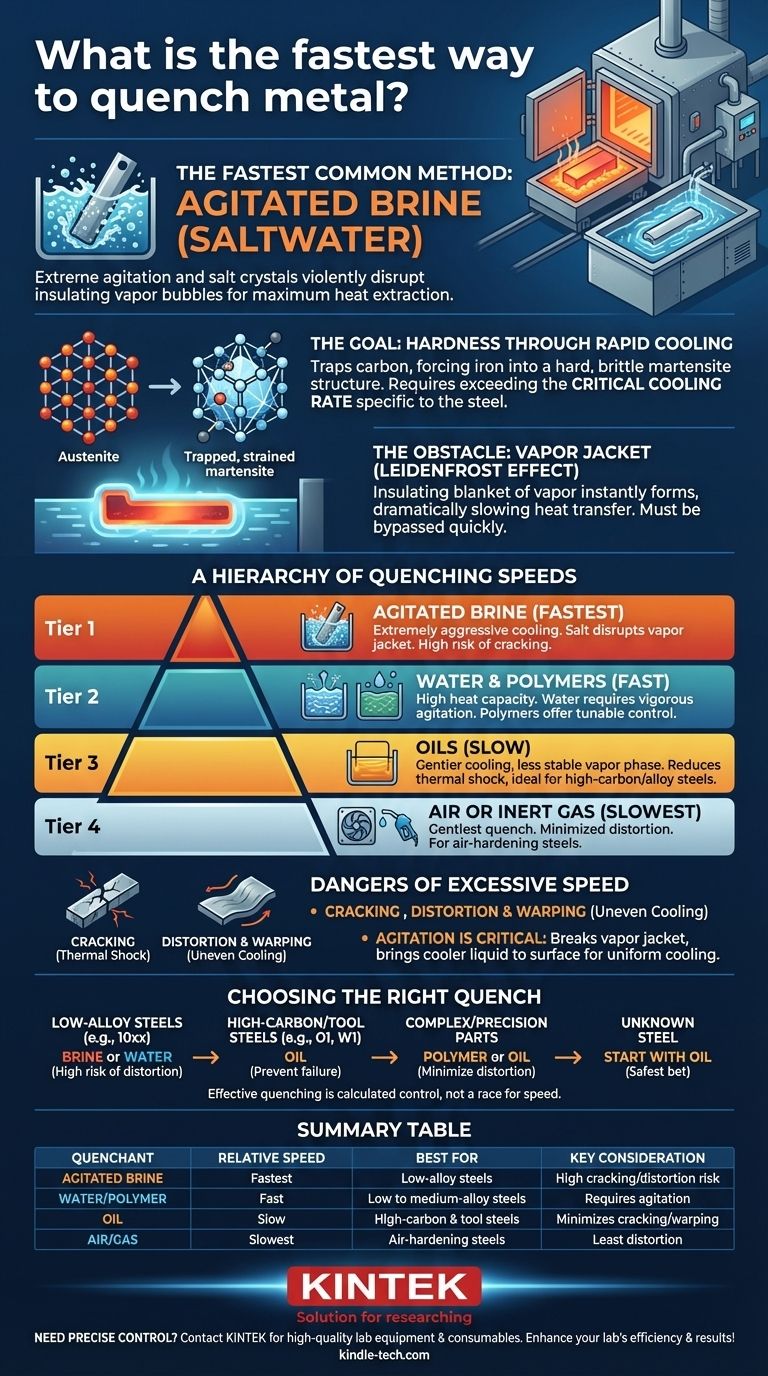
The fastest common method for quenching metal is immersing it in agitated brine (saltwater). The extreme agitation and the way salt crystals disrupt the formation of insulating vapor bubbles on the metal's surface allow for the most rapid heat extraction possible in a typical workshop or industrial setting.
While agitated brine provides the highest cooling rate, the pursuit of speed is often misguided. The true goal of quenching is to cool a specific metal just fast enough to achieve the desired hardness—and no faster—as excessive speed is the leading cause of cracking and distortion.

How Quenching Creates Hardness
To understand quench speed, you must first understand why we quench at all. The process is about trapping a specific crystal structure in the steel.
The Goal: Locking in the Martensite Structure
When you heat steel past its critical temperature (a state called austenitizing), its internal crystal structure changes to austenite, which can dissolve a large amount of carbon.
If you cool it slowly, the carbon comes out of the solution and forms soft structures like pearlite. To make steel hard, you must cool it so rapidly that the carbon atoms are trapped, forcing the iron crystals into a hard, brittle, and highly strained structure called martensite.
The Enemy: The Vapor Jacket
The single biggest obstacle to fast cooling is the Leidenfrost effect. When hot metal enters a liquid quenchant, it instantly vaporizes the liquid around it, creating an insulating blanket of vapor.
This "vapor jacket" dramatically slows down heat transfer. A successful quench depends on getting past this phase as quickly as possible.
The Key: The Critical Cooling Rate
Every type of steel has a critical cooling rate—the minimum speed required to bypass the formation of soft structures and form hard martensite. The goal is to select a quenchant that meets or slightly exceeds this rate for your specific alloy, but doesn't overshoot it so much that it introduces destructive stress.
A Hierarchy of Quenching Speeds
Quenchants are not created equal. Their ability to extract heat varies dramatically based on their physical properties.
Tier 1: Agitated Brine (The Fastest)
Brine is faster than plain water because the salt crystals violently disrupt the vapor jacket. As steam bubbles form, they immediately collapse, ensuring the liquid is always in contact with the metal surface. This provides extremely aggressive and rapid cooling.
Tier 2: Water and Polymers
Water is a very fast quenchant due to its high heat capacity. However, it is prone to forming a stable vapor jacket, which can lead to uneven cooling and soft spots if not agitated vigorously.
Polymer quenchants are a modern solution where the cooling rate can be tuned by changing the polymer concentration in water. They bridge the gap between water and oil, offering more control and reducing the risk of cracking.
Tier 3: Oils
Oils are a much slower quenchant than water. This is a deliberate feature, not a flaw. The vapor phase is less stable, and the overall cooling rate is gentler, which dramatically reduces the thermal shock to the part. This is essential for high-carbon and high-alloy steels that are very prone to cracking.
Tier 4: Air or Inert Gas
Certain high-alloy steels, known as "air-hardening" steels, have such a slow critical cooling rate that they can be hardened simply by cooling in still or forced air. This is the gentlest quench of all and results in the least amount of distortion.
Understanding the Trade-offs: Why "Fastest" is Dangerous
Selecting a quenchant that is too fast for your steel is one of the most common and costly mistakes in heat treating.
The Risk of Thermal Shock and Cracking
When you quench a part, the surface cools and shrinks almost instantly while the core remains hot and expanded. This creates immense internal stress. If the cooling rate is too extreme, this stress will exceed the material's strength, and the part will crack, often with an audible "ping."
The Problem of Warping and Distortion
Even if the part doesn't crack, uneven or excessively fast cooling can cause it to warp and distort. A perfectly machined part can become useless if it no longer meets its required dimensions after heat treatment.
The Importance of Agitation
Regardless of the quenchant, agitation is critical. Moving the part up and down or side to side (not swirling) or having a system to pump the quenchant serves two purposes: it mechanically breaks up the vapor jacket and ensures that cooler liquid is constantly being brought to the surface of the part. This promotes fast, uniform cooling.
Choosing the Right Quench for Your Steel
The optimal quenchant is a function of the steel's alloy content, the part's cross-sectional thickness, and your tolerance for distortion.
- If your primary goal is to harden simple, low-alloy steels (like the 10xx series): Brine or water may be necessary to exceed the critical cooling rate, but you must accept a higher risk of distortion or cracking.
- If your primary goal is to safely harden high-carbon or tool steels (like O1, W1, or 52100): A properly selected quenching oil is the correct choice to prevent catastrophic failure.
- If your primary goal is to minimize distortion in a complex or high-precision part: A polymer or oil quench provides the control needed to achieve hardness while preserving the part's geometry.
- If you are working with an unknown steel: Always start with the slowest quenchant (oil) first. If it doesn't harden, you can re-harden and try a faster medium, but you cannot undo a crack.
Effective quenching is not a race for speed, but a calculated control of cooling to match the specific needs of your material.
Summary Table:
| Quenchant Type | Relative Speed | Best For | Key Consideration |
|---|---|---|---|
| Agitated Brine | Fastest | Low-alloy steels | High risk of cracking/distortion |
| Water/Polymer | Fast | Low to medium-alloy steels | Requires agitation for uniformity |
| Oil | Slow | High-carbon & tool steels | Minimizes cracking and warping |
| Air/Gas | Slowest | Air-hardening steels | Least distortion, for specific alloys |
Need precise control over your heat treatment process? At KINTEK, we specialize in providing high-quality lab equipment and consumables tailored to your laboratory's needs. Whether you're working with quenching oils, polymers, or specialized furnaces, our solutions ensure you achieve the perfect hardness for your materials without the risk of cracking or distortion. Contact us today to find the right equipment for your specific steel and application – let's enhance your lab's efficiency and results together! Reach out now
Visual Guide
Related Products
- Laboratory Small Constant Temperature Heated Magnetic Stirrer Heater and Stirrer
- Professional Cutting Tools for Carbon Paper Cloth Diaphragm Copper Aluminum Foil and More
- Engineering Advanced Fine Ceramics Aluminum Oxide Al2O3 Heat Sink for Insulation
- Vacuum Arc Induction Melting Furnace
- Small Vacuum Heat Treat and Tungsten Wire Sintering Furnace
People Also Ask
- What is the temperature of sinter making? Achieve the Perfect Balance for Blast Furnace Efficiency
- What is the sintering process of stainless steel? Transform Powder into Dense, Strong Components
- What is the function of a precision isothermal heating furnace in inducing secondary phase precipitation? Optimize Microstructures
- Why is vacuum extraction combined with heating necessary in aminosiloxane synthesis? Ensure High Purity & Performance
- Why is a vacuum oven required for UIO-67 activation? Ensure Optimal Pore Clearing for Ion Conductors
- What is the purpose of using a heat treatment furnace for SiCp/2024Al composites? Master Microstructural Engineering
- What are the two methods of hardening? Through-Hardening vs. Surface Hardening Explained
- What is a resistance heating furnace? Achieve Precise, Clean High-Temperature Processing











