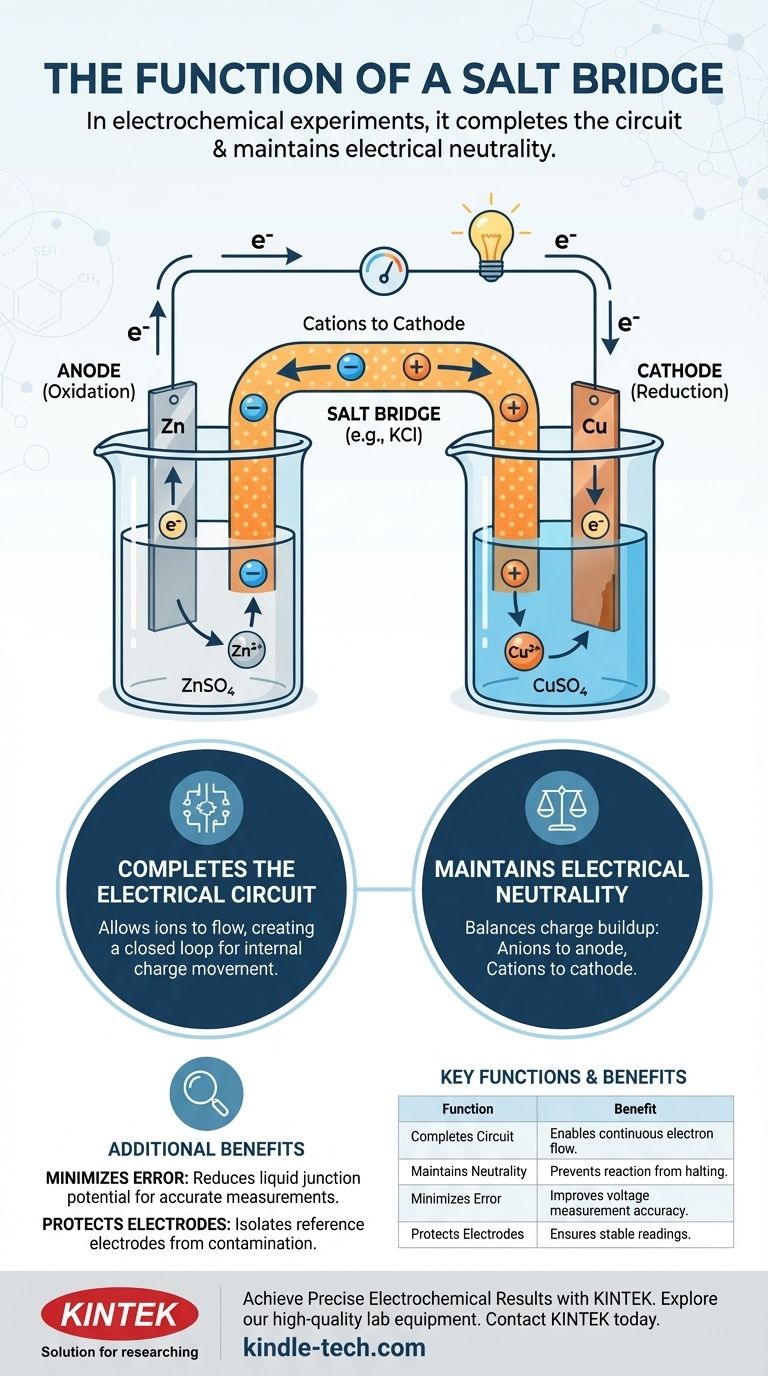
In electrochemical experiments, a salt bridge serves two primary functions: it completes the electrical circuit by allowing ions to flow between the two half-cells, and it maintains electrical neutrality in each half-cell. Without this crucial component, charge would rapidly build up, halting the flow of electrons and stopping the electrochemical reaction almost instantly.
A salt bridge is not just a connector; it is the charge-balancing mechanism that makes a sustained electrochemical reaction possible. It works internally, moving ions to counteract the charge imbalance created by electrons moving externally.

The Anatomy of an Electrochemical Cell
To understand the function of a salt bridge, you must first visualize the system it operates within. An electrochemical cell is fundamentally a device that converts chemical energy into electrical energy, or vice versa.
The Two Half-Cells
An electrochemical cell is composed of two half-cells. Each half-cell typically contains an electrode (a solid conductor like zinc or copper) submerged in an electrolyte solution (a solution containing ions, like zinc sulfate or copper sulfate).
In one half-cell, oxidation occurs (loss of electrons), and this electrode is called the anode. In the other, reduction occurs (gain of electrons), and this electrode is the cathode.
The Problem: Instant Charge Buildup
Electrons generated at the anode travel through an external wire to the cathode, creating an electrical current. However, this electron flow creates an immediate problem.
As the anode loses electrons, its electrolyte solution accumulates an excess of positive ions. Conversely, as the cathode gains electrons, its electrolyte solution develops an excess of negative ions (as positive ions from the solution are consumed). This separation of charge creates a powerful opposing voltage that brings the electron flow to a complete stop.
The Core Functions of a Salt Bridge
The salt bridge is the elegant solution to this charge buildup problem. It is typically a U-shaped tube filled with a concentrated solution of an inert electrolyte, such as potassium chloride (KCl) or potassium nitrate (KNO₃).
Completing the Electrical Circuit
A complete electrical circuit requires a closed loop. The external wire allows electrons to flow, but that's only half the circuit. The salt bridge completes the loop by allowing ions to flow between the half-cells, creating a path for charge to move internally.
Maintaining Electrical Neutrality
This is the salt bridge's most critical function. To neutralize the accumulating charge:
- Anions (negative ions) from the salt bridge migrate into the anode half-cell to balance the excess positive ions being created.
- Cations (positive ions) from the salt bridge migrate into the cathode half-cell to balance the excess negative charge.
By constantly balancing the charge in both half-cells, the salt bridge ensures the electrochemical reaction can proceed and a steady current can be maintained.
Minimizing Liquid Junction Potential
In more precise measurements, the salt bridge also serves to reduce the liquid junction potential. This is a small, unwanted voltage that forms at the interface of two different electrolyte solutions. By creating a more gradual ionic bridge, it minimizes this source of error, leading to more accurate potential measurements of the cell.
Understanding the Practical Considerations
The effectiveness of a salt bridge depends entirely on its composition and interaction with the rest of the cell.
The Electrolyte Must Be Inert
The ions within the salt bridge must not react with the ions in either half-cell solution. For example, using a KCl salt bridge in a cell containing silver nitrate (AgNO₃) would be a mistake. The chloride ions (Cl⁻) would react with the silver ions (Ag⁺) to form a solid precipitate (AgCl), disrupting the cell's function.
Protecting the Reference Electrode
In a three-electrode setup common in electrochemistry, a salt bridge is often used to connect a reference electrode (which has a constant, known potential) to the main solution. This is done to isolate the reference electrode, preventing ions from the test solution from contaminating it and altering its stable potential. This extends the electrode's service life and ensures measurement accuracy.
Making the Right Choice for Your Experiment
The primary role of the salt bridge is always to facilitate ion flow, but its importance can be viewed through different lenses depending on your goal.
- If your primary focus is a simple galvanic cell demonstration: Think of the salt bridge as the component that completes the circuit and allows the battery to run continuously.
- If your primary focus is precise voltage measurement: The salt bridge is critical for minimizing the liquid junction potential, which is a key source of experimental error.
- If your primary focus is analytical electrochemistry (e.g., corrosion studies): The salt bridge is essential for isolating your reference electrode to ensure you have a stable, reliable baseline for your potential readings.
Ultimately, the salt bridge enables the controlled and continuous study of electrochemical reactions by solving the fundamental problem of charge separation.
Summary Table:
| Function | Description | Key Benefit |
|---|---|---|
| Completes Circuit | Allows ion flow between half-cells. | Enables continuous electron flow. |
| Maintains Neutrality | Balances charge buildup from electron transfer. | Prevents reaction from halting. |
| Minimizes Error | Reduces liquid junction potential. | Improves voltage measurement accuracy. |
| Protects Electrodes | Isolates reference electrodes from contamination. | Ensures stable, reliable readings. |
Ready to achieve precise and reliable results in your electrochemical experiments? The right equipment is fundamental to your success. KINTEK specializes in high-quality lab equipment and consumables, including electrochemical cells and accessories designed for accuracy and durability. Let our experts help you select the perfect setup for your research needs.
Contact KINTEK today to discuss how we can support your laboratory's electrochemical capabilities!
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Electrolytic Electrochemical Cell with Five-Port
- Double Layer Five-Port Water Bath Electrolytic Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
People Also Ask
- How does a three-electrode electrolytic cell function? Precision Testing for 8620 Steel in Corrosive Environments
- How is a three-electrode electrochemical electrolytic cell utilized to evaluate Zr-Nb alloy corrosion resistance?
- How does an electrochemical cell system ensure measurement precision during DL-EPR? | Expert Testing Guide
- What is the volume range of the coating evaluation electrolytic cell? A Guide to Choosing the Right Size
- What are the advantages of a flat electrochemical cell for corrosion? Achieve Precise Pitting & Crevice Analysis


















