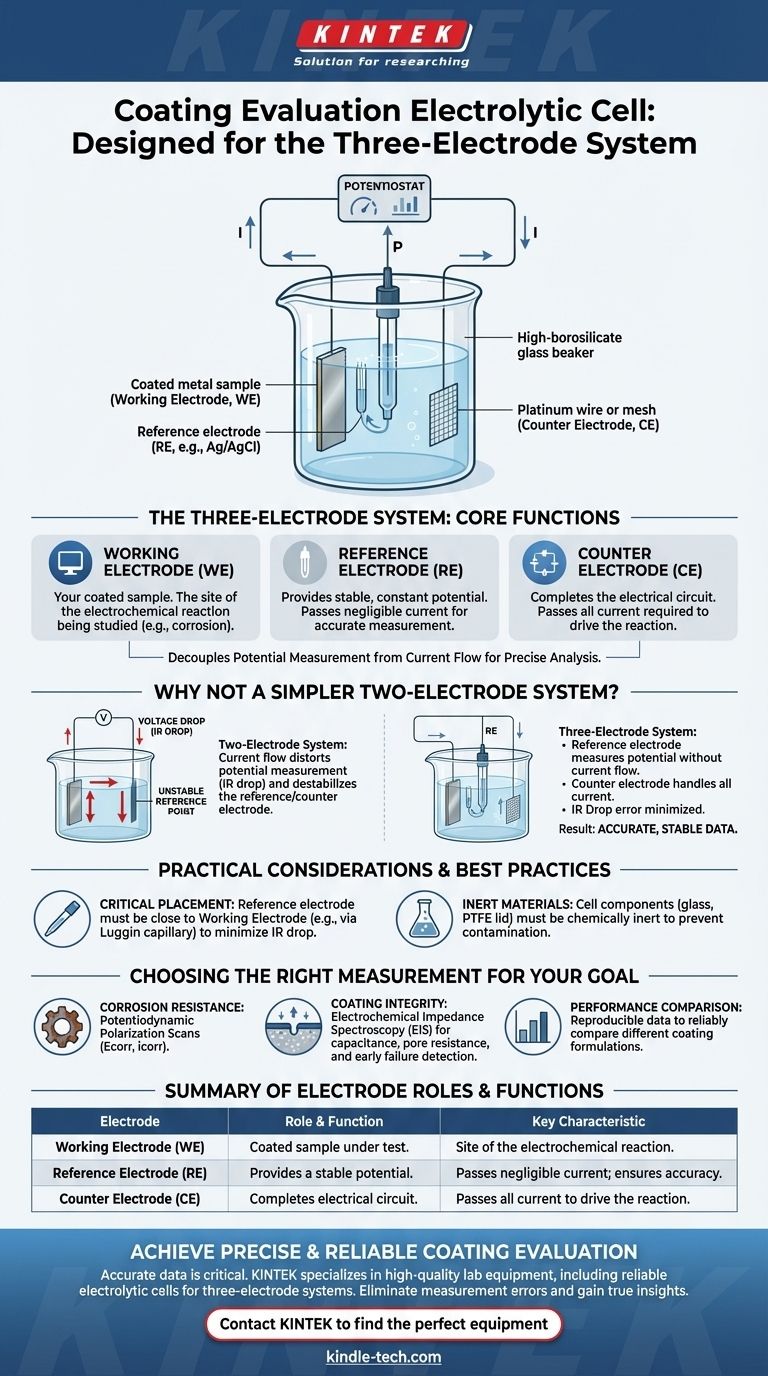
At its core, a coating evaluation electrolytic cell is specifically designed for a three-electrode system. This setup is the industry standard for obtaining accurate and reproducible electrochemical data, consisting of a working electrode, a counter electrode, and a reference electrode.
The fundamental reason for using a three-electrode system is to decouple the measurement of potential from the flow of current. This separation is what allows for a precise, stable, and accurate analysis of the coating's performance, free from the distortions that plague simpler setups.

The Purpose of a Three-Electrode System
To understand why this system is essential, you must understand the specific role each electrode plays. They work in concert, but their functions are distinct and non-interchangeable.
The Working Electrode (WE)
The working electrode is your sample of interest—the coated material you are evaluating.
This is the surface where the electrochemical reaction you want to study, such as corrosion or degradation, takes place. The goal of the entire experiment is to measure the potential and current at this electrode.
The Reference Electrode (RE)
The reference electrode is the cornerstone of measurement accuracy. It provides a stable, constant electrochemical potential.
This electrode is positioned close to the working electrode but is designed so that virtually no current flows through it. By measuring the working electrode's potential against this unchanging reference, you get a clean, reliable value.
The Counter Electrode (CE)
The counter electrode, also known as the auxiliary electrode, has one primary job: to complete the electrical circuit.
It passes all the current required by the working electrode to drive the desired reaction. By sourcing or sinking this current, it ensures the reference electrode remains undisturbed, preserving the integrity of the potential measurement.
Why Not a Simpler Two-Electrode System?
One might ask why a simpler two-electrode system isn't sufficient. The answer lies in the inherent inaccuracies of such a setup for sensitive measurements like coating evaluation.
The Problem of Voltage Drop (IR Drop)
In a two-electrode system, the same electrode acts as both the counter and the reference. As current flows between the two electrodes, a voltage drop (IR drop) occurs across the electrolyte solution.
This voltage drop gets added to the potential you are trying to measure, introducing a significant and variable error. Your measurement is no longer a pure reflection of the reaction at your working electrode.
An Unstable Reference Point
The potential of an electrode changes when current flows through it. In a two-electrode setup, because the counter/reference electrode is passing current, its own potential becomes unstable.
Measuring against a moving target makes it impossible to determine the true potential of the working electrode.
The Three-Electrode Solution
The three-electrode configuration solves both of these problems. The reference electrode measures potential without passing current, and the counter electrode passes current without being used for measurement. This elegant separation of duties is what enables precise electrochemical analysis.
Understanding the Trade-offs and Practical Considerations
While superior, the three-electrode system is not without its own set of practical requirements for achieving accurate results.
Electrode Placement is Critical
The physical placement of the electrodes matters. The reference electrode should be placed as close as possible to the working electrode to minimize any uncompensated IR drop that can still occur in the small gap of electrolyte between them. This is often achieved using a device called a Luggin capillary.
Cell Materials Must Be Inert
The materials of the electrolytic cell itself, such as high borosilicate glass for the body and Polytetrafluoroethylene (PTFE) for the lid, are chosen for their chemical inertness. This prevents the cell from reacting with the electrolyte and contaminating the experiment, which could skew the results.
System Sensitivity
This setup is highly sensitive. Results can be affected by changes in temperature, electrolyte composition and purity, and the geometric arrangement of the electrodes. Consistency across experiments is key to generating comparable data.
Making the Right Choice for Your Goal
Using a three-electrode system correctly is paramount for any serious coating analysis. Your specific goal will determine which measurements are most important.
- If your primary focus is corrosion resistance: This setup is essential for accurately performing potentiodynamic polarization scans to determine corrosion potential (Ecorr) and corrosion current (icorr).
- If your primary focus is coating integrity: Use this system for Electrochemical Impedance Spectroscopy (EIS), a powerful technique that measures coating capacitance and pore resistance to detect delamination and breakdown long before visual signs appear.
- If your primary focus is performance comparison: The stability of the three-electrode system provides the reproducible data needed to reliably compare different coating formulations or application methods.
Mastering this electrochemical setup is the foundation for generating reliable data and gaining true insight into your coating's performance.
Summary Table:
| Electrode | Role & Function | Key Characteristic |
|---|---|---|
| Working Electrode (WE) | The coated sample under test. | Site of the electrochemical reaction. |
| Reference Electrode (RE) | Provides a stable potential for measurement. | Passes negligible current; ensures accuracy. |
| Counter Electrode (CE) | Completes the electrical circuit. | Passes all current to drive the reaction. |
Ready to achieve precise and reliable coating evaluation?
Accurate electrochemical data is critical for developing durable coatings and preventing material failure. KINTEK specializes in high-quality lab equipment, including reliable electrolytic cells designed for three-electrode systems. Our solutions help you eliminate measurement errors and gain true insights into corrosion resistance and coating integrity.
Let our experts help you enhance your analysis. Contact KINTEK today to find the perfect equipment for your laboratory's needs.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Flat Corrosion Electrolytic Electrochemical Cell
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Quartz Electrolytic Electrochemical Cell for Electrochemical Experiments
People Also Ask
- What are the advantages of a flat electrochemical cell for corrosion? Achieve Precise Pitting & Crevice Analysis
- What is the operating principle of a flat plate corrosion electrolytic cell? A Guide to Controlled Materials Testing
- How does an electrochemical cell system ensure measurement precision during DL-EPR? | Expert Testing Guide
- What is the difference between electrolytic corrosion cell and electrochemical corrosion cell? Understand the Driving Force Behind Corrosion
- What role does a water-jacketed electrolytic cell play in variable-temperature electrochemical corrosion measurements?



















