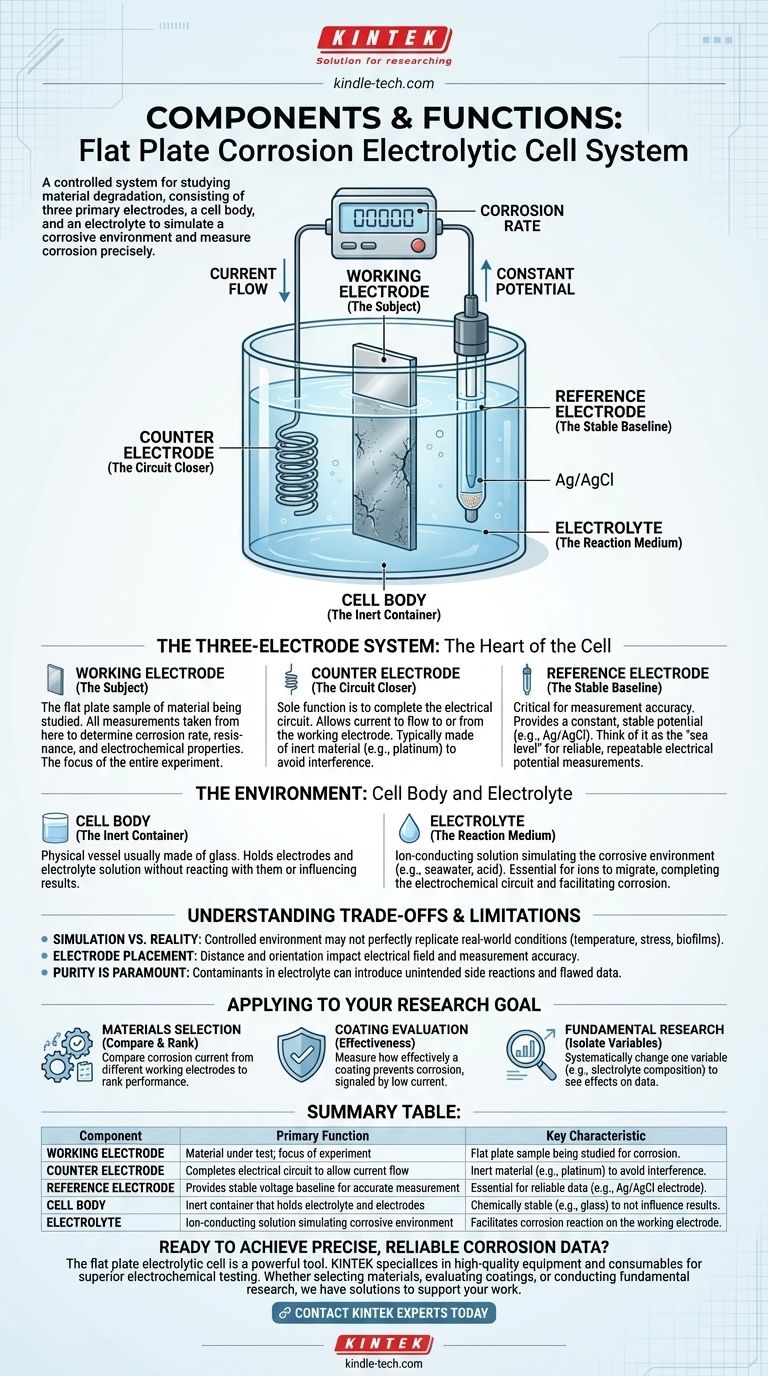
At its core, a flat plate corrosion electrolytic cell is a controlled system for studying how a material degrades. It consists of three primary electrodes (working, counter, and reference), a cell body to contain the experiment, and an electrolyte solution to simulate a corrosive environment and conduct ions.
The system is designed not just to observe corrosion, but to precisely measure it. Each component serves a distinct electrical purpose: the material under test (working electrode), a stable voltage baseline for accurate measurement (reference electrode), and a path for current to flow without interfering with the measurement (counter electrode).

The Three-Electrode System: The Heart of the Cell
The effectiveness of this setup hinges on the clear separation of roles between the three electrodes. This allows for clean, accurate data on the material's corrosion behavior.
The Working Electrode (The Subject)
The working electrode is the flat plate sample of the material you are studying. It is the focus of the entire experiment.
All measurements are taken from this component to determine its corrosion rate, resistance, and electrochemical properties.
The Counter Electrode (The Circuit Closer)
The counter electrode's sole function is to complete the electrical circuit. It allows current to flow to or from the working electrode.
It is typically made of an inert material, like platinum, to ensure it does not react or interfere with the electrochemical processes being measured at the working electrode.
The Reference Electrode (The Stable Baseline)
The reference electrode is the most critical component for measurement accuracy. It provides a constant, stable potential (voltage) that does not change during the experiment.
Think of it as the "sea level" for electrical potential. All potential measurements of the working electrode are made relative to this unwavering baseline, ensuring the data is reliable and repeatable. A common example is the silver/silver chloride (Ag/AgCl) electrode.
The Environment: Cell Body and Electrolyte
The electrodes are housed within a carefully controlled physical and chemical environment that enables the corrosion reaction to proceed in a measurable way.
The Cell Body (The Inert Container)
The cell body is the physical vessel, usually made of glass or another chemically stable material, that holds the electrodes and the electrolyte solution.
Its primary job is to contain the experiment without reacting with the electrolyte or otherwise influencing the results.
The Electrolyte (The Reaction Medium)
The electrolyte is the ion-conducting solution that surrounds the electrodes. It is designed to simulate the specific corrosive environment you want to study (e.g., seawater, acidic solution).
This solution is essential as it allows ions to migrate between the electrodes, completing the electrochemical circuit and facilitating the corrosion process on the surface of the working electrode.
Understanding the Trade-offs and Limitations
While powerful, the electrolytic cell is a laboratory model. Understanding its limitations is key to interpreting results correctly.
Simulation vs. Reality
The controlled cell environment provides excellent data but may not perfectly replicate real-world conditions, which often involve temperature fluctuations, mechanical stresses, or biological factors like biofilms.
The Importance of Electrode Placement
The geometry of the setup matters. The distance and orientation between the working, counter, and reference electrodes can significantly impact the electrical field and, consequently, the accuracy of the measurements.
Purity is Paramount
Contaminants in the electrolyte solution can introduce unintended side reactions, leading to flawed data and incorrect conclusions about the material's corrosion resistance.
Applying This to Your Research Goal
Your experimental focus will determine which aspect of the system's data is most important to you.
- If your primary focus is materials selection: You will compare the corrosion current measured from different working electrodes to rank which material performs best in a specific electrolyte.
- If your primary focus is coating evaluation: The system measures how effectively a coating on the working electrode prevents the electrolyte from causing corrosion, often signaled by a very low current.
- If your primary focus is fundamental research: You can systematically change one variable, such as the electrolyte's composition, to see how it affects the electrochemical data from the working electrode.
By isolating each electrical function, the three-electrode system empowers you to translate the complex process of corrosion into precise, actionable data.
Summary Table:
| Component | Primary Function | Key Characteristic |
|---|---|---|
| Working Electrode | The material under test; the focus of the experiment. | The flat plate sample being studied for corrosion. |
| Counter Electrode | Completes the electrical circuit to allow current flow. | Made of inert material (e.g., platinum) to avoid interference. |
| Reference Electrode | Provides a stable voltage baseline for accurate measurement. | Essential for reliable data (e.g., Ag/AgCl electrode). |
| Cell Body | An inert container that holds the electrolyte and electrodes. | Chemically stable (e.g., glass) to not influence results. |
| Electrolyte | The ion-conducting solution that simulates the corrosive environment. | Facilitates the corrosion reaction on the working electrode. |
Ready to achieve precise, reliable corrosion data for your research?
The flat plate electrolytic cell is a powerful tool, but its accuracy depends on high-quality components and expert setup. KINTEK specializes in providing the laboratory equipment and consumables you need for superior electrochemical testing.
Whether you are selecting new materials, evaluating protective coatings, or conducting fundamental corrosion research, we have the solutions to support your work.
Let's discuss your specific application. Contact our experts today to ensure your lab is equipped for success.
Visual Guide
Related Products
- Flat Corrosion Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- H Type Electrolytic Cell Triple Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- Electrolytic Electrochemical Cell with Five-Port
People Also Ask
- What are the advantages of a three-electrode flat electrolytic cell? Precise Corrosion Analysis for 1020C Steel
- What are the primary features of a flat plate corrosion electrolytic cell? Achieve Precise, Repeatable Corrosion Data
- What are the complete post-experiment procedures for a flat plate corrosion electrolytic cell? A Step-by-Step Guide to Reliable Results
- What are the design advantages of using a flat electrochemical cell? Enhance Corrosion Testing Precision
- What are the benefits of using a three-electrode flat electrochemical cell system for evaluating chromized steel?



















