At its core, corrosion is an electrochemical process, and preventing it involves disrupting that process. The two fundamental methods for protecting a metal are Barrier Protection, which physically isolates the metal from its environment, and Sacrificial Protection, which redirects the corrosion process to a different, more reactive metal.
The central choice in corrosion prevention is simple: you either put a shield between the metal and the corrosive environment, or you provide a different metal to be destroyed in its place.
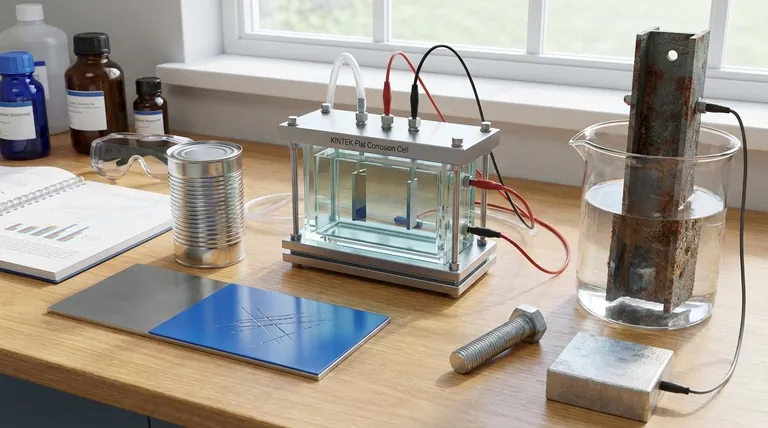
Understanding Corrosion: The Unseen Electrochemical Attack
Before stopping corrosion, we must understand what it is. Most people think of it as simple rusting, but it's a more complex electrochemical reaction.
The Role of an Electrolyte
For corrosion to occur, three things are needed: an anode (where the metal dissolves), a cathode (another part of the metal), and an electrolyte (like water or moisture) connecting them.
This setup creates a tiny electrical circuit. Electrons flow from the anode to the cathode, causing the metal at the anode to oxidize—what we see as rust or corrosion.
The Goal of Prevention
All prevention methods work by breaking this circuit. They either block the electrolyte from reaching the metal or manipulate the flow of electrons to protect the primary metal.
Method 1: Barrier Protection (Isolation)
This is the most intuitive approach: creating a physical barrier to stop oxygen and water from reaching the metal's surface. Think of it as putting a raincoat on the metal.
The Core Principle
A non-corrosive coating is applied to the metal, physically sealing it off from the surrounding environment. If the electrolyte cannot make contact, the electrochemical reaction cannot start.
Common Examples: Painting and Coating
The most common examples are paints, varnishes, and polymer coatings. These are cost-effective and provide both protection and a desired aesthetic finish for everything from cars to household appliances.
Advanced Barriers: Plating
Another form of barrier protection is plating, where a thin layer of a different, less reactive metal is coated onto the surface. For example, steel food cans are often plated with tin, and bathroom fixtures are plated with chromium for both shine and durability.
Method 2: Sacrificial Protection (Redirection)
This method is more chemically clever. Instead of just blocking corrosion, it actively redirects the electrochemical process to a different piece of metal that is intended to be destroyed.
The Core Principle
A more reactive metal is placed in electrical contact with the metal to be protected. Because it is more electrochemically active, this new metal becomes the anode and corrodes first, "sacrificing" itself to save the primary metal, which now acts as the cathode.
Common Example: Galvanization
The most widespread use of this principle is galvanizing steel, which involves coating it with a layer of zinc. Zinc is more reactive than steel (iron). When exposed to moisture, the zinc corrodes while the steel remains protected.
Large-Scale Use: Cathodic Protection
This same principle is used on a massive scale for structures like ship hulls, underground pipelines, and water heaters. Large blocks of zinc or magnesium, called sacrificial anodes, are attached to the structure. These blocks corrode away over years, protecting billions of dollars of infrastructure.
Understanding the Trade-offs
Neither method is perfect, and choosing correctly requires understanding their inherent weaknesses.
The Weakness of Barriers
The primary flaw of a barrier coating is damage. A single scratch or chip in the paint or plating exposes the underlying metal. This small breach allows corrosion to begin, and it can sometimes creep underneath the coating, causing hidden damage.
The Advantage of Sacrificial Protection
This is where sacrificial methods excel. If a galvanized steel surface is scratched, the surrounding zinc continues to protect the exposed steel. The sacrificial reaction still works as long as the two metals are in contact, making it a "self-healing" form of protection.
The Limitation of Sacrificial Metals
The main downside of sacrificial protection is that the sacrificial anode is consumed over time. It is designed to be destroyed and must eventually be replaced, which can be a significant maintenance cost for large structures.
Choosing the Right Method for Your Goal
The best approach depends entirely on the application, environment, and desired lifespan of the metal object.
- If your primary focus is aesthetics and general-purpose protection: A barrier method like high-quality paint or powder coating is usually the most effective and economical choice.
- If your primary focus is long-term durability in harsh outdoor environments: Sacrificial protection, such as galvanization, offers far more robust and reliable defense against corrosion, especially when scratches are likely.
- If your primary focus is maximum protection for a critical asset: The best strategy is often to combine both methods, such as painting over a galvanized surface to get the benefits of a barrier and the underlying security of sacrificial protection.
Ultimately, understanding these two fundamental principles empowers you to select the right defense to preserve the integrity and value of your metal assets.
Summary Table:
| Method | Core Principle | Common Examples | Key Advantage | Key Limitation |
|---|---|---|---|---|
| Barrier Protection | Isolates the metal from the environment with a physical coating. | Painting, Polymer Coatings, Chrome/Tin Plating | Cost-effective; provides aesthetic finish. | Protection fails if the coating is scratched or damaged. |
| Sacrificial Protection | Uses a more reactive "sacrificial" metal to corrode instead of the protected metal. | Galvanizing (Zinc coating), Sacrificial Anodes (on ships/pipelines) | "Self-healing"; protects even if scratched. | The sacrificial metal is consumed and must be replaced over time. |
Need to protect your laboratory equipment from corrosion?
The right corrosion prevention strategy is critical for maintaining the accuracy and longevity of your lab instruments, from autoclaves and reactors to sample holders. KINTEK specializes in providing durable lab equipment and consumables designed to withstand harsh environments.
Contact our experts today to discuss how our solutions can help you implement the most effective corrosion protection for your specific laboratory needs, ensuring your research and operations run smoothly and reliably.
Visual Guide
Related Products
- Flat Corrosion Electrolytic Electrochemical Cell
- Thin-Layer Spectral Electrolysis Electrochemical Cell
- High Purity Gold Platinum Copper Iron Metal Sheets
- Electron Beam Evaporation Coating Oxygen-Free Copper Crucible and Evaporation Boat
- Metal Disc Electrode Electrochemical Electrode
People Also Ask
- What are the complete preparation steps to be taken before using a flat plate corrosion electrolytic cell? Ensure Accurate & Repeatable Results
- What are the advantages of a three-electrode flat electrolytic cell? Precise Corrosion Analysis for 1020C Steel
- What is the difference between electrolytic corrosion cell and electrochemical corrosion cell? Understand the Driving Force Behind Corrosion
- What are the design advantages of using a flat electrochemical cell? Enhance Corrosion Testing Precision
- What is the operating principle of a flat plate corrosion electrolytic cell? A Guide to Controlled Materials Testing

















