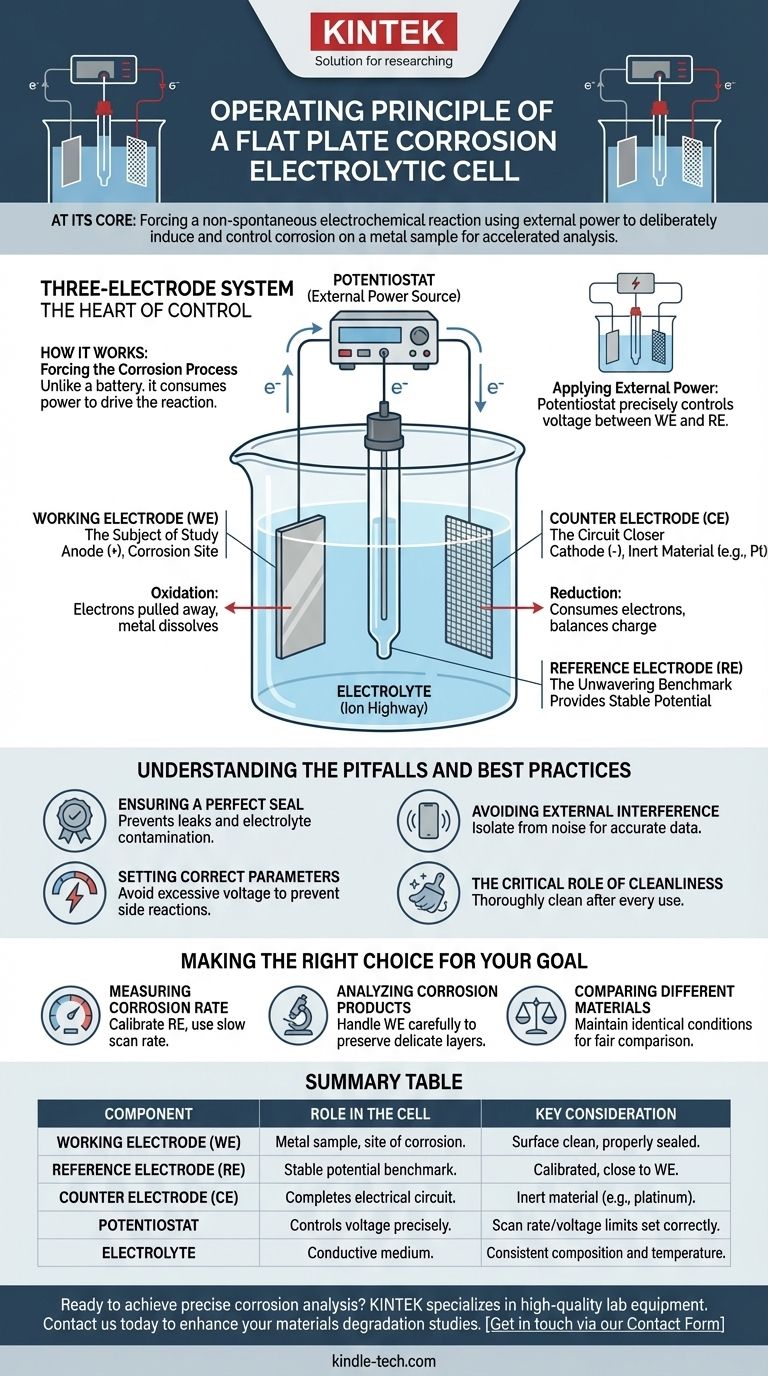
At its core, a flat plate corrosion electrolytic cell operates by using an external power source to deliberately induce and control corrosion on a metal sample. This setup forces a non-spontaneous electrochemical reaction to occur, allowing researchers to accelerate, measure, and analyze the material's degradation characteristics in a highly controlled environment.
To understand materials science, one must often study how materials fail. The flat plate electrolytic cell is a specialized tool that turns the slow, unpredictable process of corrosion into a quantifiable, repeatable experiment by using electrical energy as a precise driving force.

The Three-Electrode System: The Heart of Control
The precision of a corrosion cell comes from its three-electrode configuration. Each component has a distinct and critical function that, together, allows for accurate measurement.
The Working Electrode (WE): The Subject of Study
This is the flat plate metal sample being investigated. It is the surface where the corrosion reaction (oxidation) is intentionally initiated and measured. Its potential is the primary variable being controlled and monitored.
The Counter Electrode (CE): The Circuit Closer
Typically made of an inert material like platinum mesh, the counter electrode's sole job is to complete the electrical circuit. It allows current to flow through the electrolyte to and from the working electrode, but it does not participate in the reaction being studied.
The Reference Electrode (RE): The Unwavering Benchmark
The reference electrode, often a silver/silver chloride (Ag/AgCl) electrode, provides a stable, constant potential that does not change during the experiment. All potential measurements of the working electrode are made relative to this unwavering benchmark, ensuring the data is accurate, reproducible, and comparable across different experiments.
The Electrolyte: The Ion Highway
This is the corrosive solution (e.g., saltwater, acid) that fills the cell. It serves as a conductive medium, containing ions that migrate between the electrodes to carry charge and complete the electrochemical reaction.
How It Works: Forcing the Corrosion Process
Unlike a battery, which generates power from a spontaneous chemical reaction, an electrolytic cell consumes power to drive a reaction that would not happen on its own.
Applying External Power
An external power source, typically a sophisticated device called a potentiostat, is connected to the three electrodes. This instrument precisely controls the voltage between the working electrode and the reference electrode.
Driving Oxidation at the Anode
The potentiostat makes the working electrode the anode (the positive terminal). This electrical potential actively pulls electrons away from the metal atoms on the sample's surface, forcing them to oxidize—that is, to corrode and dissolve into the electrolyte as positive ions.
Balancing the Reaction at the Cathode
Simultaneously, the counter electrode is made the cathode (the negative terminal). It facilitates a reduction reaction (e.g., converting hydrogen ions in the electrolyte into hydrogen gas) that consumes the electrons flowing through the external circuit. This balances the overall charge.
Understanding the Pitfalls and Best Practices
While powerful, the accuracy of results from an electrolytic cell depends entirely on meticulous experimental technique.
Ensuring a Perfect Seal
The seal around the flat plate sample is a common point of failure. Any leak in the cell can compromise the electrolyte's concentration, damage the equipment, and render the experimental data invalid.
Avoiding External Interference
Corrosion measurements often involve very small electrical currents and voltages. The experimental setup must be isolated from vibrations and external electromagnetic fields (e.g., from other lab equipment) that can introduce noise and corrupt the data.
Setting Correct Parameters
Applying excessive voltage can cause unintended side reactions, such as the rapid breakdown of the electrolyte itself (electrolysis). This obscures the desired corrosion data and can even damage the electrodes. Parameters must be chosen carefully based on the material and electrolyte.
The Critical Role of Cleanliness
The cell must be thoroughly cleaned with deionized water after every use. Any residue or contamination from a previous experiment can drastically alter the chemical environment and skew the results of the next test.
Making the Right Choice for Your Goal
To get meaningful data, your experimental procedure must align with your research objective.
- If your primary focus is measuring corrosion rate (e.g., polarization curves): Ensure your reference electrode is correctly calibrated and positioned close to the working electrode, and use a slow, steady potential scan rate to allow the system to stabilize at each step.
- If your primary focus is analyzing corrosion products: Handle the working electrode with extreme care after the experiment to preserve the delicate layer of corrosion products on its surface for subsequent microscopic or spectroscopic analysis.
- If your primary focus is comparing different materials: Maintain identical experimental conditions—temperature, electrolyte composition, and electrical parameters—for every sample to ensure your comparison is fair and accurate.
By mastering these principles, you can transform the electrolytic cell from a simple piece of lab equipment into a powerful instrument for quantitative materials analysis.
Summary Table:
| Component | Role in the Cell | Key Consideration |
|---|---|---|
| Working Electrode (WE) | The metal sample under study; site of corrosion. | Surface must be clean and properly sealed to prevent leaks. |
| Reference Electrode (RE) | Provides a stable potential benchmark for accurate measurements. | Must be calibrated and positioned close to the WE. |
| Counter Electrode (CE) | Completes the electrical circuit, allowing current to flow. | Typically made of inert material like platinum. |
| Potentiostat | The external power source that controls the voltage precisely. | Scan rate and voltage limits must be set correctly to avoid side reactions. |
| Electrolyte | The corrosive solution that serves as a conductive medium. | Composition and temperature must be consistent for comparable results. |
Ready to achieve precise, repeatable corrosion analysis in your lab?
KINTEK specializes in high-quality lab equipment and consumables for materials science. Whether you are setting up a new corrosion testing station or need to optimize your existing workflow, our range of reliable electrolytic cells, electrodes, and potentiostats is designed to deliver the accuracy and control your research demands.
Contact us today to discuss your specific laboratory needs and let our experts help you select the perfect equipment to enhance your materials degradation studies.
Get in touch via our Contact Form to learn more about our products and services.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Flat Corrosion Electrolytic Electrochemical Cell
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
- Customizable PEM Electrolysis Cells for Diverse Research Applications
People Also Ask
- What are the advantages of a flat electrochemical cell for corrosion? Achieve Precise Pitting & Crevice Analysis
- How is a three-electrode electrochemical electrolytic cell utilized to evaluate Zr-Nb alloy corrosion resistance?
- How does an electrochemical cell system ensure measurement precision during DL-EPR? | Expert Testing Guide
- What is the difference between electrolytic corrosion cell and electrochemical corrosion cell? Understand the Driving Force Behind Corrosion
- How does a three-electrode electrolytic cell function? Precision Testing for 8620 Steel in Corrosive Environments



















