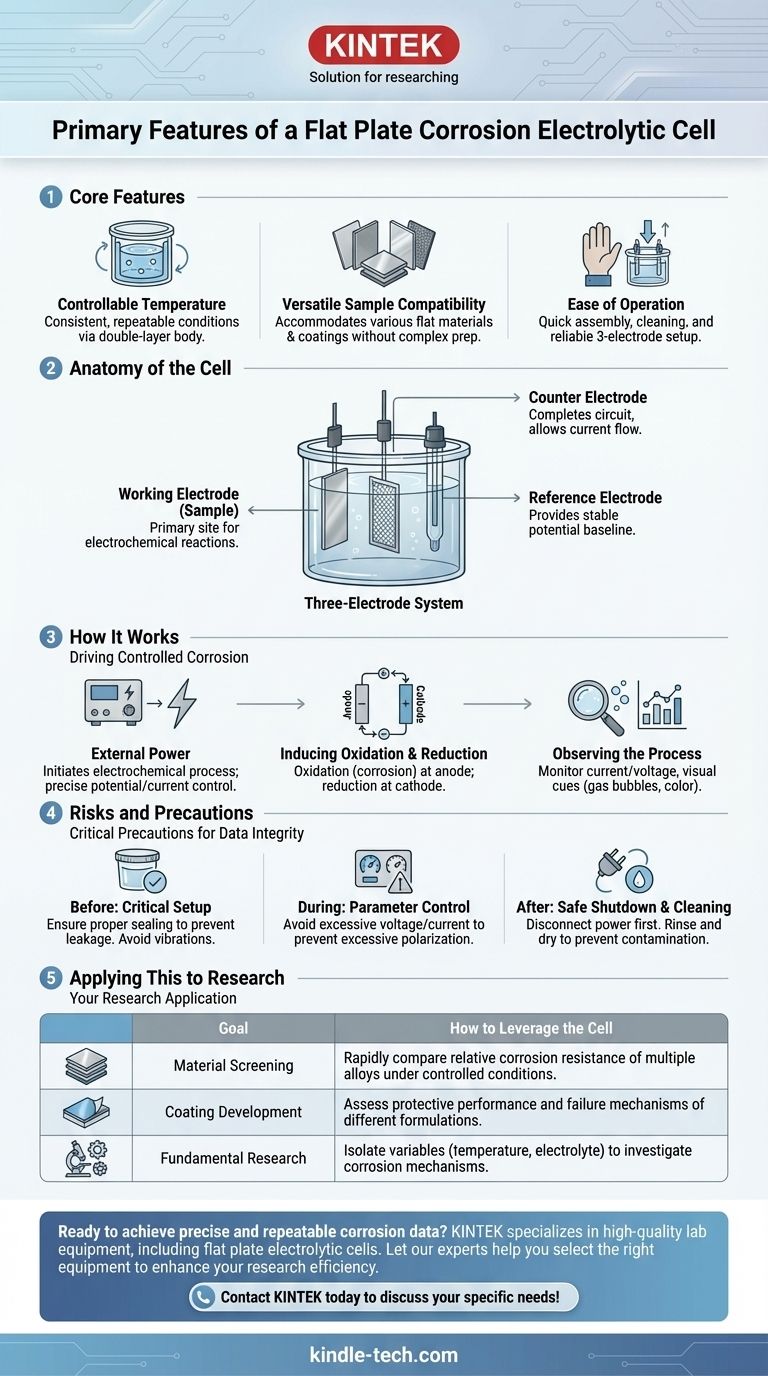
At its core, a flat plate corrosion electrolytic cell is defined by three primary features. It offers controllable temperature via a double-layer body for circulating water, versatile sample compatibility to accommodate various flat materials without complex processing, and ease of operation with a design that allows for quick assembly and cleaning.
This specialized cell is not just a container; it is a precisely controlled environment designed to produce repeatable, high-quality data for studying the electrochemical corrosion behavior of flat materials and coatings.
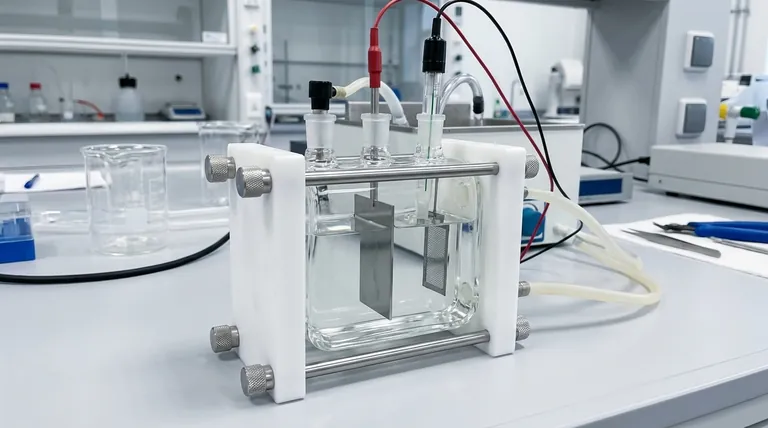
Anatomy of the Cell: The Three-Electrode System
To understand the cell's function, one must first understand its core components. The entire system is built around a standardized three-electrode configuration.
The Working Electrode (The Sample)
This is the flat plate sample being studied. It is the material on which the corrosion or coating performance evaluation takes place, acting as the primary site for the electrochemical reactions of interest.
The Counter Electrode (Completing the Circuit)
The counter electrode, often a platinum mesh, serves to complete the electrical circuit. It allows current to flow through the electrolyte to or from the working electrode without interfering with the measurement.
The Reference Electrode (The Stable Baseline)
A reference electrode, such as a silver chloride electrode, provides a constant, stable potential. All potential measurements of the working electrode are made relative to this unchanging baseline, ensuring accurate and comparable data.
The Cell Body and Electrolyte (The Environment)
The cell body, typically made of glass, contains the electrolyte solution. This solution acts as a medium for ion migration, creating the specific chemical environment required for the corrosion reaction to occur.
How It Works: Driving Controlled Corrosion
Unlike a galvanic cell where a reaction occurs spontaneously, an electrolytic cell uses external energy to drive a non-spontaneous reaction. This control is the key to its scientific value.
The Role of External Power
An external power source is connected to the cell, supplying the electrical energy needed to initiate the electrochemical process. This allows researchers to precisely control the potential or current applied to the sample.
Inducing Oxidation and Reduction
The applied energy forces electrochemical reactions. Oxidation (loss of electrons, i.e., corrosion) occurs at the anode, while reduction (gain of electrons) occurs at the cathode. By controlling the system, the flat plate sample is made to act as the working electrode where these processes can be studied.
Observing the Corrosion Process
As the experiment runs, researchers can monitor changes in current and voltage. Visual cues like gas bubbles (indicating a gas evolution reaction) or color changes (signaling corrosion product formation) provide additional qualitative data.
Understanding the Risks and Precautions
While powerful, the flat plate cell requires careful handling to ensure data integrity and operator safety. Mismanagement can lead to flawed results and potential hazards.
Before You Begin: Critical Setup
Always ensure the cell is properly sealed to prevent electrolyte leakage, which can compromise data and pose a safety risk. The experimental environment should be free from vibrations or electromagnetic fields that could interfere with sensitive measurements.
During the Experiment: Parameter Control
Set power parameters carefully based on your materials. Excessive voltage or current can cause excessive electrode polarization, potentially damaging the cell or losing experimental control, rendering the data useless.
After the Experiment: Safe Shutdown and Cleaning
Always disconnect the power source first. When disassembling, handle electrodes with care to preserve any corrosion products for later analysis. The cell and components must be thoroughly rinsed, typically with deionized water, and dried completely before storage to prevent cross-contamination.
How to Apply This to Your Research
Your specific goal determines how you leverage the cell's capabilities.
- If your primary focus is material screening: Use the cell to rapidly compare the relative corrosion resistance of multiple alloys or materials under identical, controlled conditions.
- If your primary focus is coating development: The cell is ideal for assessing the protective performance and failure mechanisms of different coating formulations on a standardized substrate.
- If your primary focus is fundamental research: Leverage the cell’s precise control to isolate variables and investigate how factors like temperature, electrolyte composition, or potential affect corrosion mechanisms.
This tool provides a standardized window into the complex world of electrochemical corrosion.
Summary Table:
| Primary Feature | Key Benefit |
|---|---|
| Controllable Temperature | Ensures consistent, repeatable test conditions via a double-layer water jacket. |
| Versatile Sample Compatibility | Accommodates various flat materials and coatings without complex preparation. |
| Ease of Operation | Allows for quick assembly, cleaning, and reliable three-electrode system setup. |
Ready to achieve precise and repeatable corrosion data in your lab?
KINTEK specializes in high-quality lab equipment, including corrosion testing cells. Our flat plate electrolytic cells are designed to provide the controllable environment you need for accurate material screening, coating development, and fundamental research.
Let our experts help you select the right equipment to enhance your research efficiency and data quality.
Contact KINTEK today to discuss your specific laboratory needs!
Visual Guide
Related Products
- Flat Corrosion Electrolytic Electrochemical Cell
- Electrolytic Electrochemical Cell for Coating Evaluation
- Electrolytic Electrochemical Cell with Five-Port
- PTFE Electrolytic Cell Electrochemical Cell Corrosion-Resistant Sealed and Non-Sealed
- H-Type Double-Layer Optical Electrolytic Electrochemical Cell with Water Bath
People Also Ask
- What are the complete preparation steps to be taken before using a flat plate corrosion electrolytic cell? Ensure Accurate & Repeatable Results
- What are the components and their respective functions in a flat plate corrosion electrolytic cell system? A Guide to Precise Corrosion Measurement
- What are the complete post-experiment procedures for a flat plate corrosion electrolytic cell? A Step-by-Step Guide to Reliable Results
- What is a flat cell for corrosion testing? Achieve Non-Destructive, In-Situ Analysis
- What are the advantages of a three-electrode flat electrolytic cell? Precise Corrosion Analysis for 1020C Steel



















