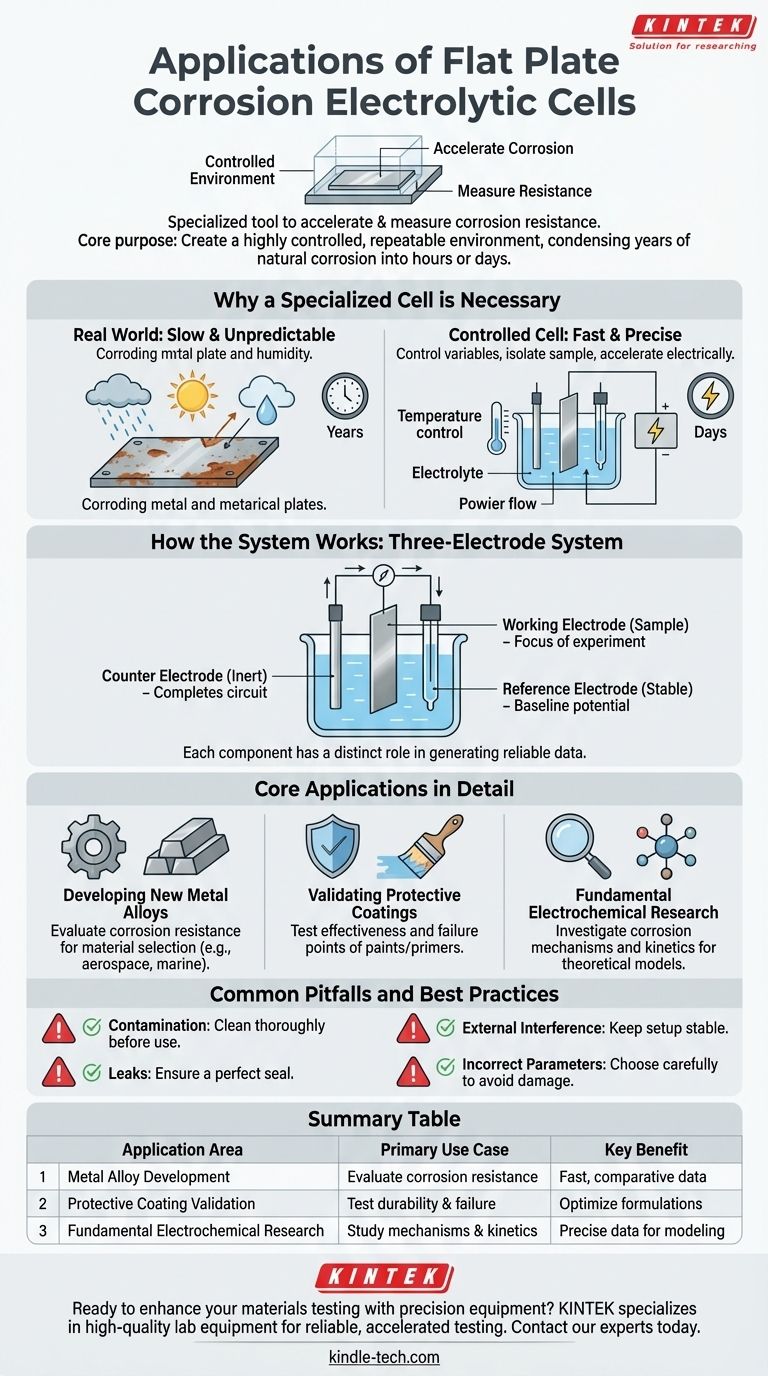
In short, a flat plate corrosion electrolytic cell is a specialized laboratory tool used to accelerate and measure the corrosion resistance of flat materials. Its primary applications are in metal materials research, the performance testing of protective coatings, and fundamental electrochemical corrosion studies.
The core purpose of a flat plate corrosion cell is to create a highly controlled and repeatable environment. This allows researchers to precisely simulate and measure how a material will degrade over time, condensing years of natural corrosion into a matter of hours or days.

Why a Specialized Cell is Necessary
To understand corrosion, you must be able to control the variables that cause it. A flat plate cell provides a standardized framework to study how materials behave in specific, aggressive environments.
The Challenge of Studying Corrosion
Corrosion in the real world is slow and unpredictable. It is influenced by countless factors like humidity, temperature, and chemical exposure, making it difficult to study systematically.
Creating a Controlled Environment
The cell isolates a flat sample in a specific electrolyte solution, which acts as the corrosive medium. A double-layered cell body often allows for temperature control via circulating water, ensuring the test conditions remain constant.
Accelerating the Process Electrically
By connecting the sample (the working electrode) and other electrodes to an external power source, researchers can manipulate electrical potential. This drives the electrochemical reactions that cause corrosion, dramatically speeding up the process.
How the System Works
The cell operates as a three-electrode system, where each component has a distinct and critical role in generating reliable data.
The Working Electrode (The Sample)
This is the flat piece of material being tested. It is the focus of the experiment, and its surface is where the corrosion reactions are measured.
The Counter Electrode
Typically made of an inert material like platinum, the counter electrode completes the electrical circuit. It allows current to flow through the electrolyte to the working electrode without interfering with the reaction being studied.
The Reference Electrode
The reference electrode, such as a silver/silver chloride electrode, provides a stable, constant potential. All potential measurements of the working electrode are made relative to this unchanging baseline, ensuring accuracy and repeatability.
The Electrolyte and Cell Body
The electrolyte is the liquid medium that conducts ions and simulates the corrosive environment (e.g., saltwater). The cell body, usually made of glass, simply contains the system and ensures it is sealed.
Core Applications in Detail
The controlled nature of the flat plate cell makes it invaluable for specific, high-stakes research and development goals.
Developing New Metal Alloys
Engineers use the cell to evaluate the corrosion resistance of new alloys. By comparing how different formulations perform under identical conditions, they can select the best materials for demanding applications like aerospace or marine construction.
Validating Protective Coatings
The cell is essential for testing the effectiveness of paints, primers, and other protective coatings. It can determine how quickly a coating breaks down and fails, helping to optimize the coating process and formulation for maximum durability.
Fundamental Electrochemical Research
Academics and scientists use the cell to investigate the underlying mechanisms of corrosion. By precisely controlling factors like voltage and electrolyte composition, they can gather data to build theoretical models that explain why and how materials degrade.
Common Pitfalls and Best Practices
While powerful, the accuracy of a flat plate cell experiment depends entirely on meticulous procedure and an awareness of potential sources of error.
Contamination and Improper Cleaning
The cell and electrodes must be thoroughly cleaned before each use. Any residue from previous experiments can contaminate the electrolyte and invalidate the results.
Preventing Leaks
A perfect seal is non-negotiable. Electrolyte leakage not only compromises the experiment's data but also poses a safety hazard to the operator and can damage equipment.
Avoiding External Interference
Electrochemical measurements are highly sensitive. The experimental setup must be kept stable and free from external vibrations or electromagnetic fields, which can introduce noise and affect the data.
Setting Correct Parameters
Applying excessive voltage or current can damage the sample or the cell and lead to misleading results. Experimental parameters must be chosen carefully based on the specific material and electrolyte being used.
Applying This to Your Goal
Your specific objective determines how you interpret the results from a flat plate corrosion cell.
- If your primary focus is material development: Use the cell to rank the relative corrosion performance of different alloy candidates in a simulated service environment.
- If your primary focus is coating validation: Use the cell to measure the failure rate of a coating and identify its weakest points for future improvement.
- If your primary focus is academic research: Use the cell to collect precise current and voltage data to support or challenge existing theories of corrosion kinetics.
Ultimately, this tool transforms the slow, complex process of corrosion into a measurable and predictable science.
Summary Table:
| Application Area | Primary Use Case | Key Benefit |
|---|---|---|
| Metal Alloy Development | Evaluate corrosion resistance of new materials | Fast, comparative data for material selection |
| Protective Coating Validation | Test the durability and failure points of paints/primers | Optimize coating formulations for longevity |
| Fundamental Electrochemical Research | Study the mechanisms and kinetics of corrosion | Generate precise data for theoretical modeling |
Ready to enhance your materials testing with precision equipment?
KINTEK specializes in high-quality lab equipment, including corrosion testing cells, to meet the demanding needs of research and development laboratories. Our flat plate corrosion electrolytic cells provide the controlled environment you need for reliable, accelerated testing of metals and coatings.
Let us help you achieve accurate and repeatable results. Contact our experts today to discuss your specific application and find the perfect solution for your lab.
Visual Guide
Related Products
- Electrolytic Electrochemical Cell for Coating Evaluation
- Customizable PEM Electrolysis Cells for Diverse Research Applications
- Customizable CO2 Reduction Flow Cell for NRR ORR and CO2RR Research
- Laboratory Test Sieves and Sieving Machines
- Customizable XRD Sample Holders for Diverse Research Applications
People Also Ask
- What type of electrode system is the coating evaluation electrolytic cell designed for? Unlock Precise Coating Analysis
- What is the operating principle of a flat plate corrosion electrolytic cell? A Guide to Controlled Materials Testing
- How is a high-precision electrolytic cell used to evaluate metal corrosion resistance? Validate DCT Results Accurately
- What is the volume range of the coating evaluation electrolytic cell? A Guide to Choosing the Right Size
- How does a three-electrode electrolytic cell function? Precision Testing for 8620 Steel in Corrosive Environments


















